One-time treatment with lifileucel (Amtagvi, Iovance Biotherapeutics, Inc.) continues to show deep, durable responses and meaningful survival in patients with advanced melanoma, according to a five-year analysis of the C-144-01 trial.
The study was presented at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, IL, and simultaneously published in the Journal of Clinical Oncology.
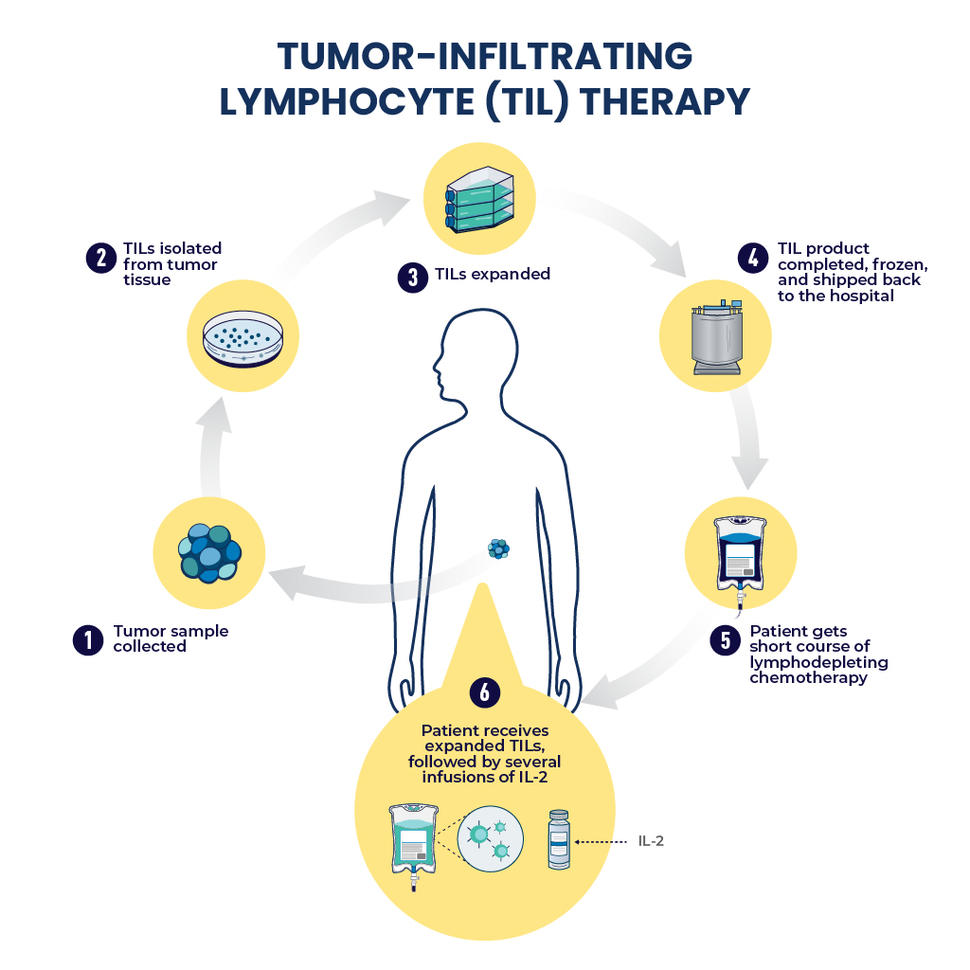
Lifileucel is the first individualized tumor-infiltrating lymphocyte (TIL) therapy. It uses a patient’s own immune cells (TILs) to target and destroy cancer cells. This five-year analysis of the C-144-01 trial includes advanced melanoma patients previously treated with anti-PD-1 and targeted therapy, where applicable. The analysis includes 153 patients from cohorts 2 and 4 of the C-144-01 trial.
Key Takeaways
Key takeaways from the study include:
- Meaningful five-year overall survival rate of 20% in patients with advanced melanoma previously treated with immune checkpoint inhibitors.
- At a median follow-up of 57.8 months, the median overall survival (OS) was 13.9 months, with 19.7% of patients surviving at the five-year mark.
- The objective response rate was 31.4%, with a median time to response of 1.4 months and a median duration of response of 36.5 months.
- Nearly one-third of responders (31.3%) completed the five-year assessment with ongoing responses.
The observed safety profile was consistent with that of nonmyeloablative lymphodepletion and interleukin-2 administration. The incidence of adverse events (AEs) decreased rapidly within the first two weeks after lifileucel infusion, and there were no new or late-onset treatment-related AEs.
“Amtagvi has demonstrated long-term benefit and meaningful overall survival in a difficult-to-treat melanoma patient population resistant to immune checkpoint inhibitor therapy,” says Theresa Medina, MD, Medical Oncologist at the University of Colorado Cancer Center on the Anschutz Medical Campus in Aurora, CO, in a news release. “Five years following one-time Amtagvi treatment, responses persisted or deepened during an extended treatment-free interval for some patients. Amtagvi offers a new standard of care for the advanced melanoma community and sets a new bar for one-time cell therapies with curative intent in solid tumors.”
Accelerated Approval
In February 2024, the U.S. Food and Drug Administration (FDA) granted accelerated approval to lifileucel for the treatment of adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor. Iovance is also conducting TILVANCE-301, a Phase 3 trial in frontline advanced melanoma, to confirm clinical benefit.
IMAGE CAPTION: Tumor-infiltrating lymphocytes, or TILs, are T cells collected from a patient’s own tumor. Once isolated from the tumor sample, the TILs are expanded into the billions and infused back into the patient.
IMAGE CREDIT: National Cancer Institute