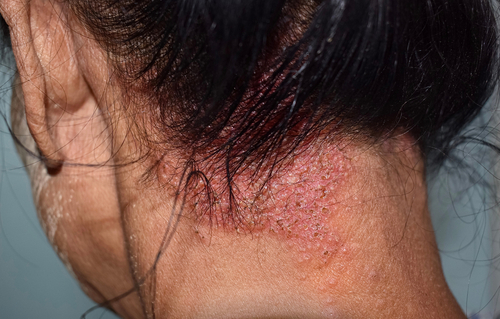
Roflumilast foam 0.3% (Zoryve, Arcutis) performed well as a once-daily monotherapy treatment for psoriasis of the scalp and body, according to new Phase 3 data in the Journal of American Medical Association (JAMA).
Treatment with roflumilast foam resulted in significant improvements across multiple efficacy endpoints, including the co-primary efficacy endpoints of Scalp-Investigator Global Assessment (S-IGA) Success and Body-Investigator Global Assessment (B-IGA) Success, as well as key secondary endpoints. The data also show improvement in pruritus as early as 24 hours after the first application.
Roflumilast foam is under review for the treatment of scalp and body psoriasis by the U.S. Food and Drug Administration (FDA) with a Prescription Drug User Fee Act (PDUFA) target action date of May 22, 2025.
“These compelling results demonstrate that ZORYVE foam 0.3% may provide rapid and significant relief of plaques anywhere on the body and is well-tolerated according to both investigator and patient-reported assessments,” says study author Melinda Gooderham, MD, MSc, FRCPC, Dermatologist and Medical Director at the SKiN Centre for Dermatology, and Principal Investigator at the SKiN Research Centre in Ontario, Canada, in a news release. “The foam formulation is particularly beneficial for its versatility in treating hair-bearing and non-hair-bearing skin, which could ultimately help patients adhere to their treatment.”
About the ARRECTOR Study
A Randomized tRial Employing topiCal roflumilasT foam to treat scalp psORiasis (ARRECTOR), was a Phase 3, randomized, double-blinded, vehicle-controlled trial which enrolled 432 adults and adolescents aged 12 years and older with plaque psoriasis affecting the scalp and body, across 49 sites in the United States and Canada.
Significantly greater proportions of individuals treated with roflumilast foam 0.3% achieved the co-primary efficacy endpoints of S-IGA Success and B-IGA Success, defined as an IGA score of ‘clear’ or ‘almost clear’ plus a 2-point improvement from baseline. At Week 8, 66.4% of individuals treated with roflumilast foam 0.3% achieved S-IGA success compared to 27.8% for vehicle. At Week 8, 45.5% of patients treated with roflumilast foam achieved B-IGA success compared to 20.1% for vehicle.
Key Findings
Other key findings include:
- Roflumilast foam provided a clinically meaningful improvement in scalp itch. 65.3% of individuals treated with roflumilast foam achieved a clinically significant reduction in itch compared to 30.3% of individuals treated with vehicle at Week 8 as measured by a ≥ 4-point change from baseline in Scalp Itch-Numeric Rating Scale (SI-NRS). Significant improvement was seen in the roflumilast foam treatment group as early as Week 2. The data also demonstrated improvement in body itch as measured by the Worst Itch-Numeric Rating Scale (WI-NRS) at Week 8, with 63.1% of those treated with roflumilast foam 0.3% achieving a ≥ 4-point reduction in WI-NRS compared to 30.1% of those treated with vehicle. Significant improvement was seen in the roflumilast foam treatment group as early as Week 2.
- Importantly, there was a greater improvement in itch observed with roflumilast foam within 24 hours after the first application compared to vehicle. This improvement over vehicle within 24 hours after the first application was also observed in body itch.
- At Week 8, 70.9% of those treated with roflumilast foam versus 31.3% treated with vehicle achieved at least 75% improvement in Psoriasis Scalp Severity Index (PSSI-75), another secondary endpoint.
- Similarly, 50.1% of people treated with roflumilast foam 0.3% achieved at least 75% improvement in Psoriasis Area and Severity Index (PASI-75), a key secondary endpoint, as compared to 16.8% of those treated with vehicle at Week 8.
Well-Tolerated Treatment
Roflumilast foam was well-tolerated. The incidence of Treatment Emergent Adverse Events (TEAEs) was low and similar in both active treatment and vehicle arms. The most frequent adverse events in the roflumilast foam arm (≥1%) included headache, diarrhea, and nausea. Investigator-rated application-site tolerability was similar between Roflumilast foam and vehicle groups, with investigators reporting no evidence of irritation for at least 99.2% of all patients at all time points. Patient-rated application-site tolerability was also similar between roflumilast foam and vehicle with at least 94.4% of patients reporting no or mild sensation on local tolerability assessments at all time points.
“We are thrilled that JAMA Dermatology, a premier medical journal, has published the positive results of our pivotal ARRECTOR study. These data demonstrate that investigational ZORYVE foam rapidly relieved psoriasis symptoms, including the most troublesome symptom of itch,” adds Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis. “As an effective, safe, and well-tolerated once-daily treatment, ZORYVE foam 0.3%, if approved, will offer those living with psoriasis a potential new treatment option for use anywhere on the body with no limitations on duration of use. Having a single agent that effectively treats psoriasis on both the scalp and body, with a safe and tolerable profile, is an important therapeutic benefit for both clinicians and the people they treat. We look forward to the FDA’s potential approval of ZORYVE foam 0.3% for the treatment of plaque psoriasis of the scalp and body anticipated later this month.”
Next-Generation Topical
Roflumilast foam is a next-generation topical phosphodiesterase-4 (PDE4) inhibitor. The 0.3% topical foam is indicated for treatment of seborrheic dermatitis in adult and pediatric patients aged 9 and older.
The 0.3% cream is approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients aged 6 and older. The 0.15% cream is approved by the FDA for the topical treatment of mild to moderate atopic dermatitis in patients aged 6 and up. The 0.05% cream is under review for the treatment of atopic dermatitis in children ages 2 to 5 by the FDA, with a PDUFA target action date of October 13, 2025.