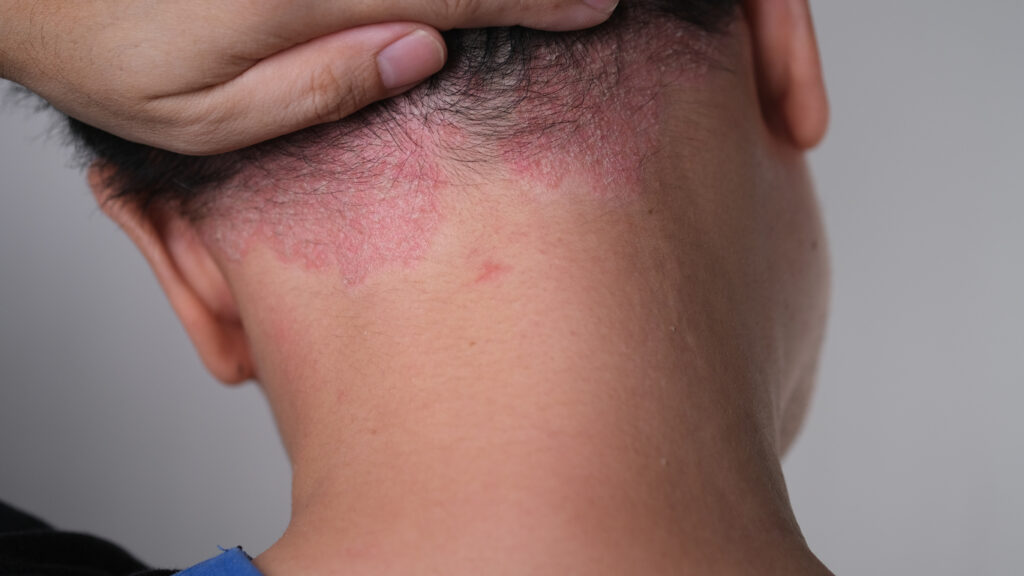
Arcutis Biotherapeutics submitted a supplemental new drug application (sNDA) to the U.S. Food and Drug Administration (FDA) for roflumilast foam 0.3% (ZORYVE), a once-daily, next generation phosphodiesterase-4 (PDE4) inhibitor, for the treatment of adults and adolescents ages 12 and older with scalp and body psoriasis.
In clinical trials, roflumilast foam 0.3% was effective and well-tolerated, with almost 70% of patients treated with roflumilast foam achieving Scalp-Investigator Global Assessment (S-IGA) Success at Week 8. Roflumilast foam 0.3% is already FDA approved for the treatment of seborrheic dermatitis and roflumilast cream 0.3% is already approved for plaque psoriasis. Roflumilast cream was recently approved for mild to moderate atopic dermatitis in patients aged six years and older.
“Nearly half of all individuals with plaque psoriasis have involvement of the scalp. Hair-bearing areas present unique treatment challenges that are not easily addressed with creams or ointments. Individuals with plaque psoriasis need steroid-free treatment options that clear plaques anywhere on the body and relieve burdensome symptoms, such as itch and flaking,” says clinical trial investigator Melinda Gooderham, MSc, MD, FRCPC, Medical Director of SKiN Centre for Dermatology in Peterborough, Ontario, Canada and Investigator with Probity Medical Research, in a news release.
“Clinical data show that investigational once-daily ZORYVE foam effectively and reliably cleared both scalp and body psoriasis across all efficacy endpoints compared to vehicle, including 67% of participants achieving Scalp-Investigator Global Assessment Success with ZORYVE foam at 8 weeks. These data demonstrate that ZORYVE foam, if approved, would be an important new treatment option for those living with psoriasis.”
The submission is supported by positive results from Arcutis’ pivotal ARRECTOR Phase 3 trial, a Phase 2b study, and long-term efficacy and safety data generated from the ZORYVE cream development program in plaque psoriasis.
The “A Randomized tRial Employing topiCal roflumilasT foam to treat scalp psORiasis” (ARRECTOR) study was a parallel group, double-blind, vehicle-controlled pivotal Phase 3 study of the safety and efficacy of roflumilast foam 0.3% or a matching vehicle administered once-daily in individuals with plaque psoriasis of the scalp and body ages 12 and older (n=432). The study met its co-primary endpoints with 67.3% of individuals treated with roflumilast foam achieving Scalp-Investigator Global Assessment (S-IGA) Success (IGA Success is defined as an IGA score of ‘clear’ or ‘almost clear’ plus a 2-point improvement from baseline) compared to 28.1% of individuals treated with a matching vehicle foam at Week 8, and 46.5% of individuals treated with roflumilast foam achieved Body-Investigator Global Assessment (B-IGA) Success compared to 20.8% of individuals treated with a matching vehicle foam at Week 8.
Two thirds (65.3%) of roflumilast-treated patients with clinically meaningful itch at baseline achieved a clinically significant reduction in itch compared to 30.3% of vehicle-treated patients at Week 8 as measured by a ≥ 4-point change from baseline in Scalp Itch-Numeric Rating Scale (SI-NRS). Importantly, some patients experienced rapid relief in scalp itch 24 hours following first application compared to vehicle (as measured by mean SI-NRS change from baseline P=0.0164).
In addition, improvement in body itch as measured by the Worst Itch-Numeric Rating Scale (WI-NRS) was also observed at Week 8, with 63.1% of those treated with roflumilast foam achieving a ≥ 4-point reduction in WI-NRS compared to 30.1% of those treated with vehicle, as well as Week 2 (the earliest timepoint measured).
Roflumilast foam 0.3% was well tolerated. The incidence of Treatment Emergent Adverse Events (TEAEs) was low and generally similar between active treatment and vehicle, with most TEAEs assessed as mild to moderate severity. Overall, the most common adverse reactions for roflumilast foam in the phase 3 and phase 2b studies (≥1%) included headache (3.1%), diarrhea (2.5%), nausea (1.7%), and nasopharyngitis (1.3%). Rates of discontinuation due to adverse events were low and similar among roflumilast-treated (1.8%) and vehicle-treated (1.3%) patients.