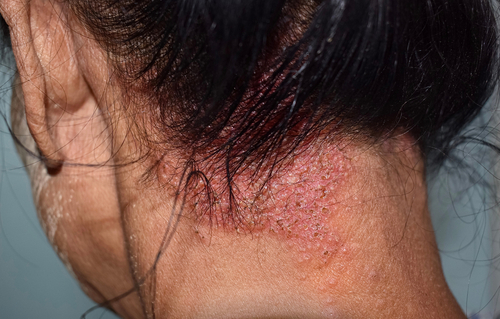
Roflumilast foam, 0.3% (Zoryve, Arcutis) bests vehicle in the treatment of seborrheic dermatitis, with 80% of individuals achieving Investigator Global Assessment (IGA) success and 51% of individuals reaching complete clearance at Week 8, according to new research published in the February 2024 issue of the Journal of American Academy of Dermatology.
Zoryve foam was approved by the U.S. Food and Drug Administration (FDA) for treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older in December 2023. It is the first drug approved for seborrheic dermatitis with a new mechanism of action in over two decades.
The STudy of Roflumilast foam Applied Topically for the redUction of seborrheic derMatitis (STRATUM) was a parallel group, double-blind, vehicle-controlled study evaluating the safety and efficacy of Zoryve (roflumilast) foam, 0.3% in seborrheic dermatitis. The trial enrolled 457 adults and adolescents with moderate to severe seborrheic dermatitis affecting up to 20% body surface area (BSA), including the scalp, face, trunk, and/or intertriginous areas.
The STRATUM study met its primary endpoint, with 80% of roflumilast foam treated individuals reaching IGA Success rate at Week 8 (79.5% ZORYVE foam vs. 58.0% vehicle; IGA Success was defined as an IGA score of clear or almost clear plus a ≥2 grade improvement from baseline.
Improvement was seen early, with roflumilast foam demonstrating a statistically significant improvement compared to vehicle on IGA Success at Week 2, the first timepoint assessed in STRATUM. In addition, 50.6% of individuals in the roflumilast foam treated arm reached complete clearance (IGA=0) at Week 8.
Roflumilast foam also demonstrated statistically significant improvement over vehicle on all secondary endpoints, including itch, scaling, and erythema (redness). More than 60% of individuals achieved a ≥4-point reduction in itch at Week 8 as measured by Worst Itch-Numerical Rating Score (62.8% roflumilast foam vs. 40.6% vehicle), and significant improvements in itch were also reported at Week 2 and Week 4.
Individuals treated with Zoryve foam reported a 28% improvement in itch from baseline in 48 hours (compared to 13% on vehicle nominal p=0.0024). More than 50% of individuals treated with ZORYVE foam achieved an erythema (redness) score of 0, and more than 50% achieved a scaling score of 0, at Week 8.
Treatment with Zoryve foam demonstrated a significantly larger improvement in patient reported outcomes as early as Week 2 as measured through Dermatology Life Quality Index (DLQI), with improvements maintained through Week 8.
Zoryve foam was well-tolerated with a favorable safety and tolerability profile. The incidence of Treatment Emergent Adverse Events (TEAEs) was low and similar between active treatment and vehicle, with most TEAEs assessed as mild to moderate severity. There were no treatment-related Serious Adverse Events (SAEs). The most common adverse reactions (≥1%) reported, per the prescribing information, include nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
“Despite being very common, seborrheic dermatitis has traditionally been a disease with limited treatment options. It also can have a significant impact on quality of life,” says study author Andrew Blauvelt, MD, MBA, investigator at the Oregon Medical Research Center in Portland, in a news release. “The publication of the Phase 3 STRATUM study results in the Journal of American Academy of Dermatology further validates the significance of roflumilast foam as a new treatment option for seb derm, one that provides treatment success in eight of ten patients, along with significant and rapid improvements in key signs and symptoms of disease, as early as two weeks. These results highlight the effectiveness and safety of roflumilast foam, a steroid-free treatment and the first novel mechanism of action approved for seb derm in two decades. It should end suffering from this long-neglected condition.”
“Seborrheic dermatitis is challenging to manage, and often people with the disease require several different treatments for different areas of their body. Our focus has been to simplify the treatment of seborrheic dermatitis with an effective once-daily steroid-free foam that is suitable for use anywhere on the body for any duration and on all hair and skin types,” adds Patrick Burnett, MD, PhD, chief medical officer of Arcutis. “We are pleased that this manuscript is now available to provide dermatology clinicians with a greater understanding of the clinical data supporting ZORYVE foam as a newly approved treatment option for their patients.”
Matthew Zirwas, MD, a dermatologist in Bexley, Ohio, is excited about this new option.“This is the first thing for seborrheic dermatitis that works quickly, consistently, and is safe and approved for daily long term use,” he says in an interview with TDD.
“What is most fascinating to me about roflumilast foam is that in the psoriasis study, it worked BETTER on the scalp than on other areas, which is unheard of. We see so many people with difficult to treat seb derm or sebopsoriasis of the scalp, and we haven’t had great options – this, in my opinion, is the most effective topical we’ve ever had for psoriasis and seborrheic dermatitis of the scalp.“
Most of the people Dr. Zirwas treated in the trials tell him that their disease is gone, and his is supported by the data, which showed that about 80% of people got to clear or almost clear. “My definition of “almost clear” is that a patient can’t tell they still have any active disease but a dermatologist can still appreciate it on close inspection,’ he says. “The takeaway is that…when we prescribe roflumilast foam for seborrheic dermatitis, we can tell patients that 8 out 10 people who use it can’t tell that they have seborrheic dermatitis after a month or so of using it.”