Arcutis Biotherapeutics, Inc. announced the closing of its previously announced underwritten public offering of 18,157,895 shares of common stock at a public offering price of $9.50 per share.
This includes 2,368,421 shares issued upon the exercise in full by the underwriters of their option to purchase additional shares of common stock at the public offering price, less underwriting discounts and commissions.
The total gross proceeds of the public offering were approximately $172.5 million, before deducting underwriting discounts and commissions and offering expenses payable by Arcutis.
All of the shares in the public offering were sold by Arcutis.
Morgan Stanley, TD Cowen, and Guggenheim Securities acted as joint lead bookrunning managers for the offering. Mizuho and Needham & Company acted as co-lead managers for the offering.
The shares in the public offering were offered by Arcutis pursuant to an effective shelf registration statement on Form S-3 that was filed with the Securities and Exchange Commission (the “SEC”) on January 31, 2024.
A final prospectus supplement and accompanying prospectus relating to and describing the final terms of the offering has been filed with the SEC and is available on the SEC’s website located at http://www.sec.gov or may be obtained from Morgan Stanley & Co. LLC, Attention: Prospectus Department, 180 Varick Street, 2nd Floor, New York, NY 10014, or by email at prospectus@morganstanley.com; Cowen and Company, LLC, 599 Lexington Avenue, New York, NY 10022, by telephone at (833) 297-2926, or by email at Prospectus_ECM@cowen.com; or Guggenheim Securities, LLC Attention: Equity Syndicate Department, 330 Madison Avenue, 8th Floor, New York, NY 10017, by telephone at (212) 518-9544, or by email at GSEquityProspectusDelivery@guggenheimpartners.com.
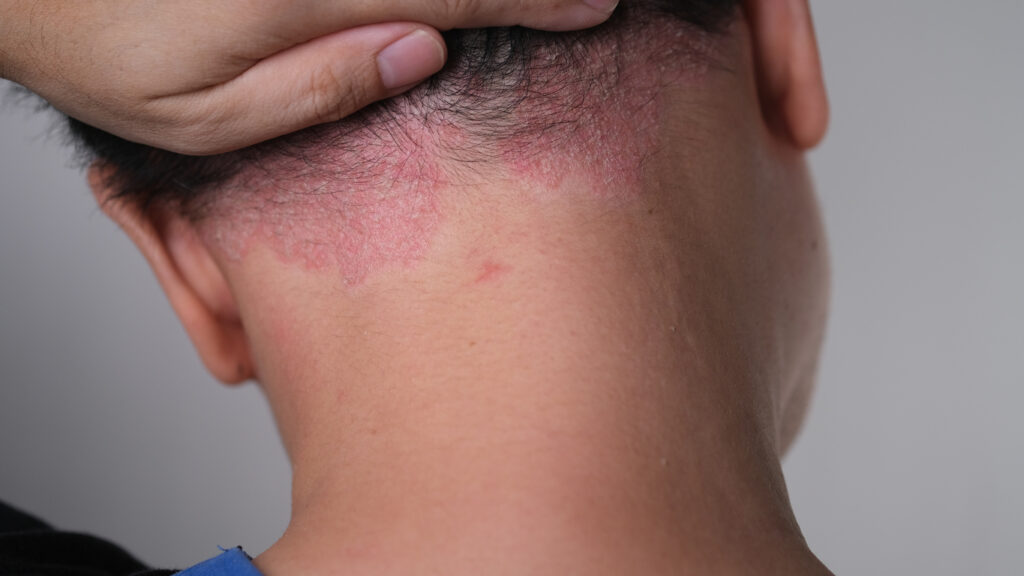
The U.S. Food and Drug Administration (FDA) approved topical roflumilast foam (Zoryve, Arcutis) for seborrheic dermatitis. The PDE4 inhibitor is the first topical treatment for seborrheic dermatitis with a new mechanism of action in more than 20 years.