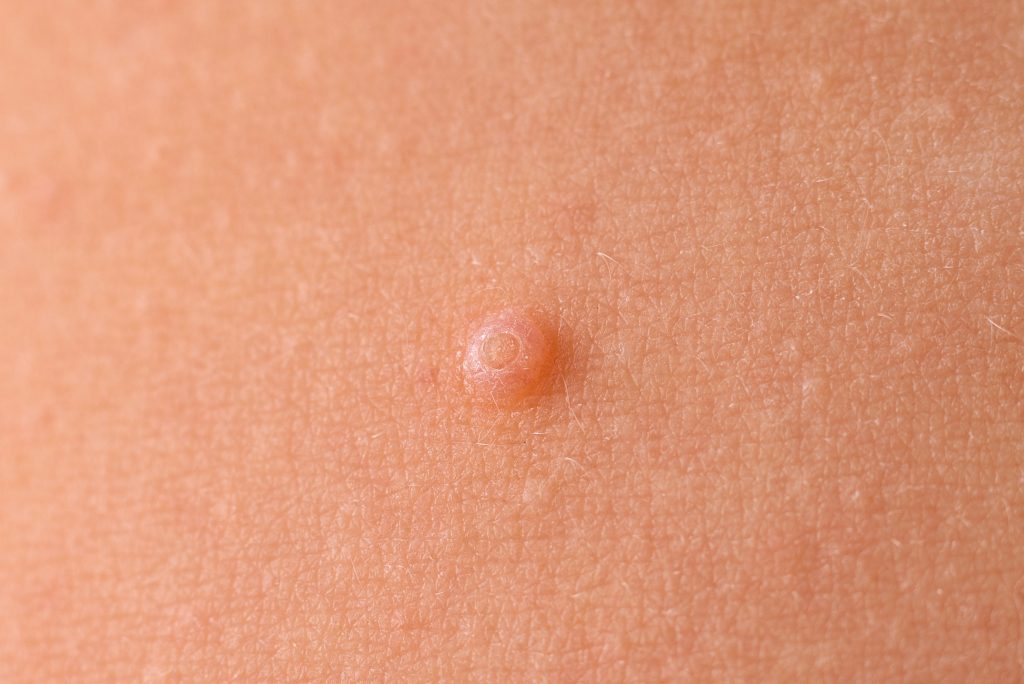
The U.S. Food and Drug Administration (FDA) has approved Zelsuvmi (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum in adults and children one year of age and older.
Zelsuvmi is expected to be commercially available during the second half of 2024, according to Ligand Pharmaceuticals.
Zelsuvmi is the first novel drug for the treatment of molluscum infections and the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home, outside of a physician’s office, or other medical setting to treat molluscum contagiosum.
For years, there were no FDA approved treatments for molluscum contagiosum, and now there are two. Approved in 2023 Verrica Pharmaceuticals’ Ycanth (cantharidin) was the first U.S.-approved treatment for this infection, but, unlike Zelsuvmi, it requires medical supervision during application. Cantharidin was regularly used off-label for the treatment of molluscum contagiosum before Ycanth was approved by the FDA.
Zelsuvmi is a nitric oxide releasing agent. Nitric oxide has been shown to have antiviral properties. The precise mechanism of action for Zelsuvmi in the treatment of molluscum contagiosum is unknown.
“The approval of Zelsuvmi a breakthrough, marking the first time that clinicians can treat molluscum with an efficacious topical prescription medication that is applied by the patient, or a family member,” says Mark D. Kaufmann, MD, a Clinical Professor of Dermatology in the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City and Past President of the American Academy of Dermatology, in a news release. “I look forward to having this novel medication to treat my molluscum patients.”
When asked how he sees Zelsuvmi fitting in to the molluscum treatment paradigm, Dr. Kaufmann told The Derm Digest that it will likely be combined with in-office procedures.
Zelsuvmi’s efficacy was demonstrated in 2 Phase 3 trials – B-SIMPLE 4 and B-SIMPLE 2. These trials showed Zelsuvmi’s ability to reduce lesion counts and was well tolerated when used once a day. The B-SIMPLE Phase 3 program enrolled 1,598 patients.
The most commonly reported adverse reactions (≥1%) in clinical trials were application site reactions.
“Pediatricians, dermatologists, and caregivers have long-sought a convenient approach to treat this highly contagious skin infection. With Zelsuvmi, patients now have an at-home treatment option available,” says Todd Davis, CEO of Ligand, in a news release.
Ligand Pharmaceuticals acquired all the assets of Novan, Inc. in September 2023. Novan developed Zelsuvmi. On July 17, 2023, Ligand offered $15 million to acquire all of the assets of Novan as well as to provide up to $15 million in debtor-in-possession financing. In September 2023, the Bankruptcy Court approved a $12.2 million bid from Ligand to purchase berdazimer gel and all the assets related to the Nitricil technology platform, and the rights to the Bayer-partnered Sitavig program.
Dr. Kaufmann is a consultant for Novan/Ligand.