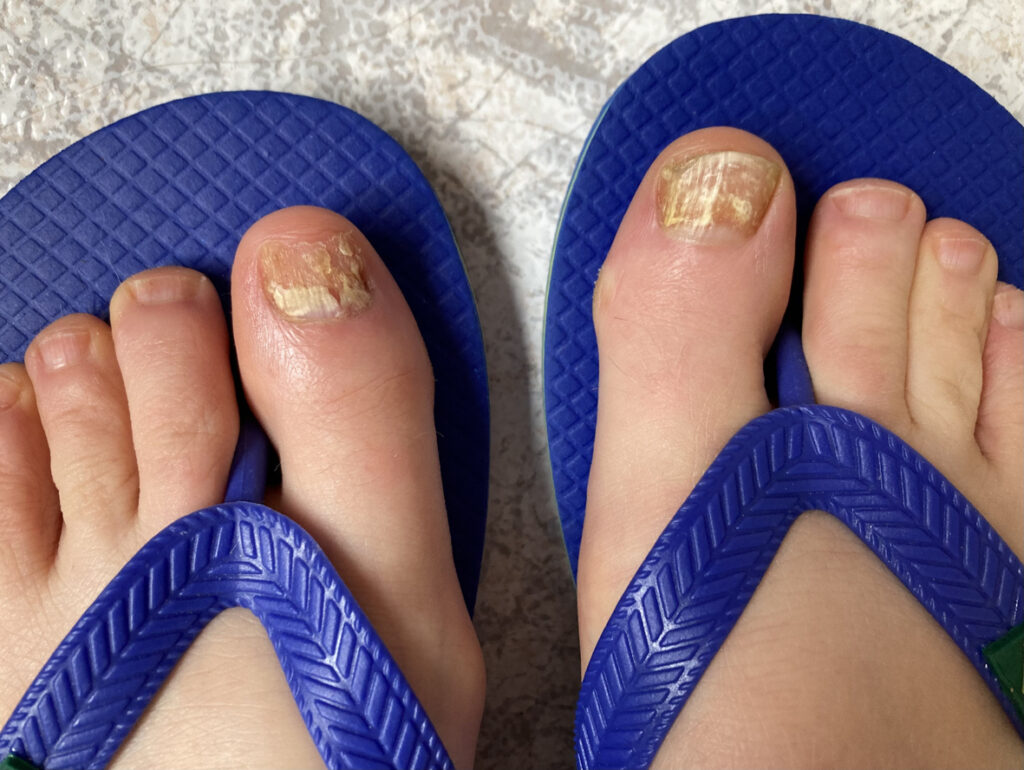
Almirall, S.A. has completed the decentralized regulatory procedure in Europe for efinaconazole (Jublia), a triazole antifungal compound indicated for onychomycosis .
The successful completion of this procedure is the final step before national marketing authorizations can be granted by European countries. Work with the national regulatory authorities is now underway, and marketing authorizations are expected within H1 2025, the Company reports,
In July 2021, Kaken and Almirall entered into a license and distribution agreement, granting Almirall the exclusive rights for the development and commercialization of this topical formulation in Europe.
The product has been marketed in Japan since 2014 under the trade name Clenafin. It has also been launched in the United States, Canada, Korea, Hong Kong, and Macau by Kaken Pharmaceutical’s partner companies under the trade name Jublia.