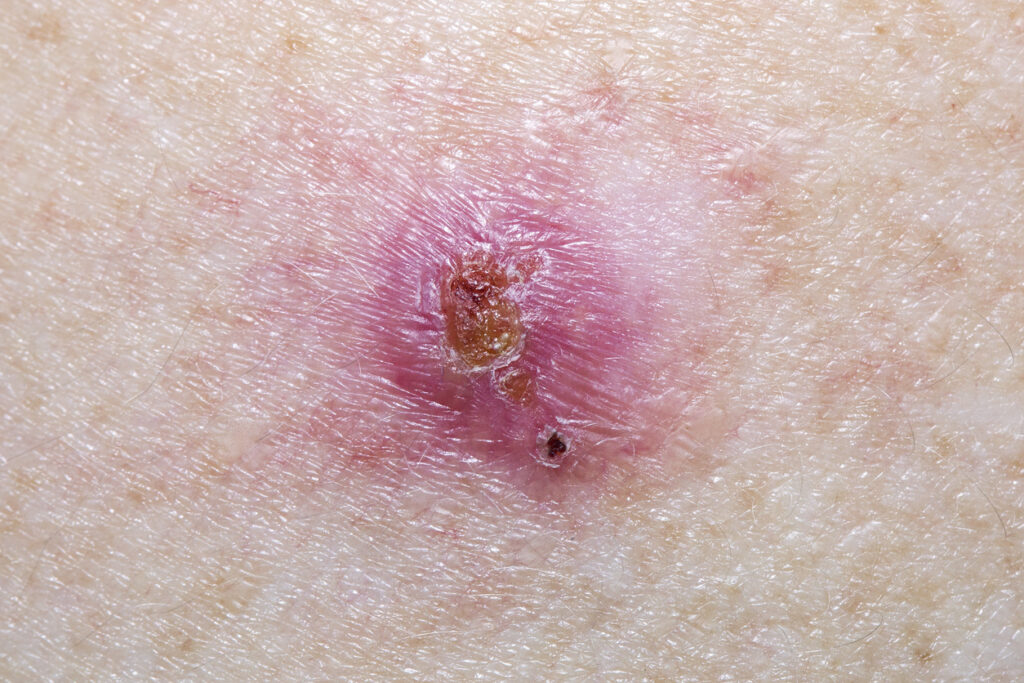
The U.S. Food and Drug Administration (FDA) has given AiViva Biopharma its OK to test AIV001 in facial skin by intradermal injection for nonmelanoma skin cancer.
AIV001 is currently in development for evaluation in superficial and nodular superficial basal cell carcinoma.
AIV001 is a pan-tyrosine kinase inhibitor in a proprietary formulation designed for prolonged drug release. This FDA clearance was supported by AiViva’s submission of safety data on non-facial skin tolerability and efficacy, and systemic exposure from 67 subjects treated with AIV001 in basal cell carcinoma and in scar management.
“This agreement allows a clear path forward to develop AIV001 in cosmetically sensitive skin that includes the face, neck, and scalp, and sets it on track to be the first therapeutic developed for facial skin areas having sBCC or nBCC lesions,” says Diane Tang-Liu, PhD, President and CEO of AiViva, in a news release. “We are excited about the product prospect of AIV001 using a focal delivery method that is supported by our strong intellectual property position.”
To date, AiViva has completed three clinical studies using intradermal injection of AIV001. Subjects received one to three treatments, three weeks apart. The potency of AIV001 and prolonged drug release in the skin provided clinical and histological clearance of biopsied-confirmed basal cell carcinoma lesions in 26 patients. In addition, a total of 41 subjects were treated in two trials using scar models. Reduced fibrosis formation was observed after a single intradermal injection over the surgical incisional wounds and indicated AIV001’s potential use in scar management. “This was an important achievement for the clinical development of AIV001 allowing us to expand our ability to evaluate AIV001’s utility in dermatological conditions,” adds Darlene Deecher, AiViva’s Vice President of Clinical Development.