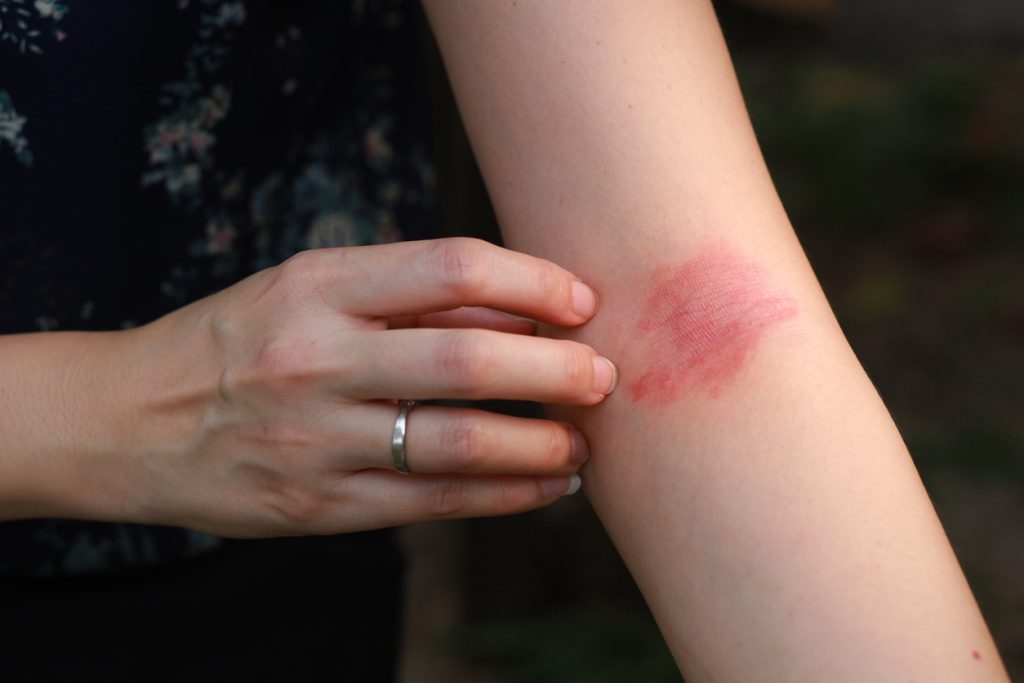
The U.S. Food and Drug Administration (FDA) has granted Fast Track designation for Nektar Therapeutics’ rezpegaldesleukin for the treatment of adult and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Rezpegaldesleukin is an investigational biologic therapy that targets the interleukin-2 receptor complex to stimulate proliferation of inhibitory immune cells known as regulatory T cells.
FDA’s Fast Track designation is granted to investigational therapies that treat serious conditions and have the potential to address an unmet medical need. A drug candidate that receives Fast Track designation is eligible for more frequent meetings and written interactions with the FDA to discuss the drug candidate’s development plan as well as possible eligibility for rolling review and priority review.
In patients with moderate-to-severe AD, rezpegaldesleukin has been shown to rapidly improve measurable exploratory disease outcomes during a 12-week induction treatment phase and for at least 36 weeks after ceasing treatment, demonstrating proof-of-concept in this indication. Proof-of-concept efficacy and safety data from a Phase 1b study of rezpegaldesleukin in AD patients were presented at the 2023 European Academy of Dermatology and Venereology Congress.
“We are pleased that rezpegaldesleukin has been designated a Fast Track product,” says Jonathan Zalevsky, Ph.D., Senior Vice President and Chief Research & Development Officer at Nektar, in a news release. “Rezpegaldesleukin has the potential to address a significant unmet need for the millions of patients living with moderate-to-severe atopic dermatitis. We remain on track to announce topline data from the induction period of our Phase 2b REZOLVE-AD study in the second quarter of this year. This designation will now allow us to collaborate closely with the agency on the design of the registrational program for rezpegaldesleukin once we’ve completed Phase 2.”