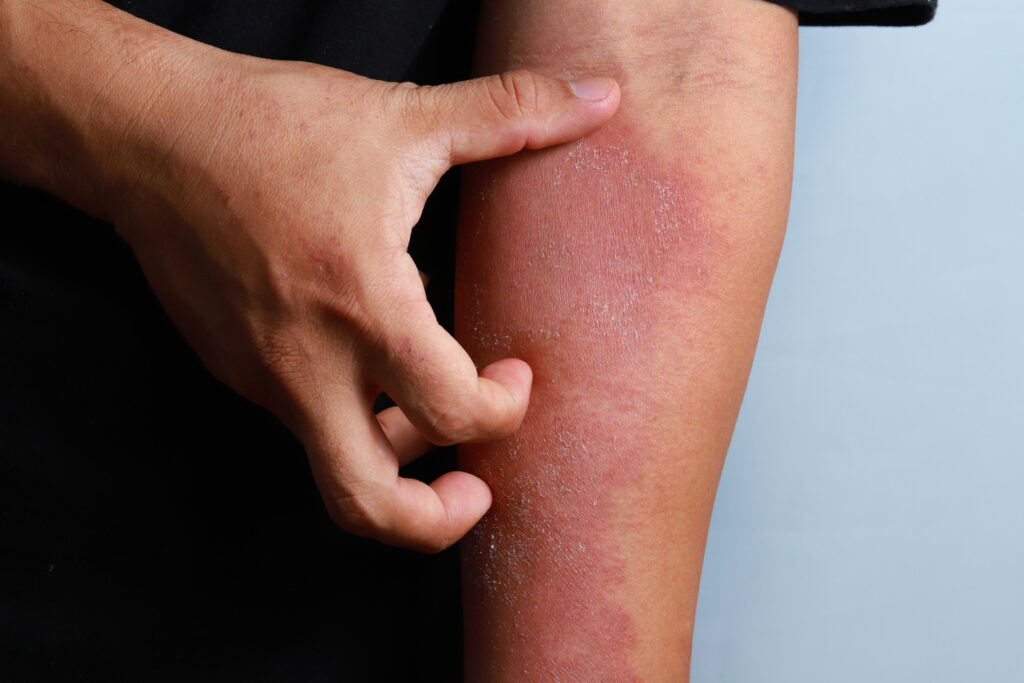
Evommune, Inc. has enrolled the first patient in a Phase 2 trial of EVO301, an injectable interleukin (IL)-18 neutralizer, in moderate-to-severe atopic dermatitis (AD).
EVO301 is a serum albumin Fab-associated interleukin (IL)-18BP fusion protein designed to neutralize upregulated IL-18 activity, which is implicated in a range of inflammatory and autoimmune diseases.
The multi-center, randomized, double-blind, placebo-controlled, proof-of-concept trial is designed to evaluate the safety and efficacy of EVO301 in approximately 60 adult patients with moderate-to-severe AD. The primary objective of this study is to characterize the efficacy of EVO301, assessed by the percentage change in EASI from baseline at week 12. EASI, the Eczema Area and Severity Index score, is a tool used to measure the extent and severity of atopic dermatitis.
“AD is a highly heterogenous disease, and for this reason, there remains a need for more effective treatments. While currently available therapies may improve some signs and symptoms, many AD patients do not respond optimally. We believe EVO301 could, if approved, fulfill their search for a new and better treatment option. Based on our robust preclinical and Phase 1 clinical data, we are eager to confirm that the extended half-life expected with this IL-18BP fusion protein has the potential to better serve patients with moderate-to-severe AD,” says J. Mark Jackson, MD, Vice President, Clinical Development at Evommune, in a news release.