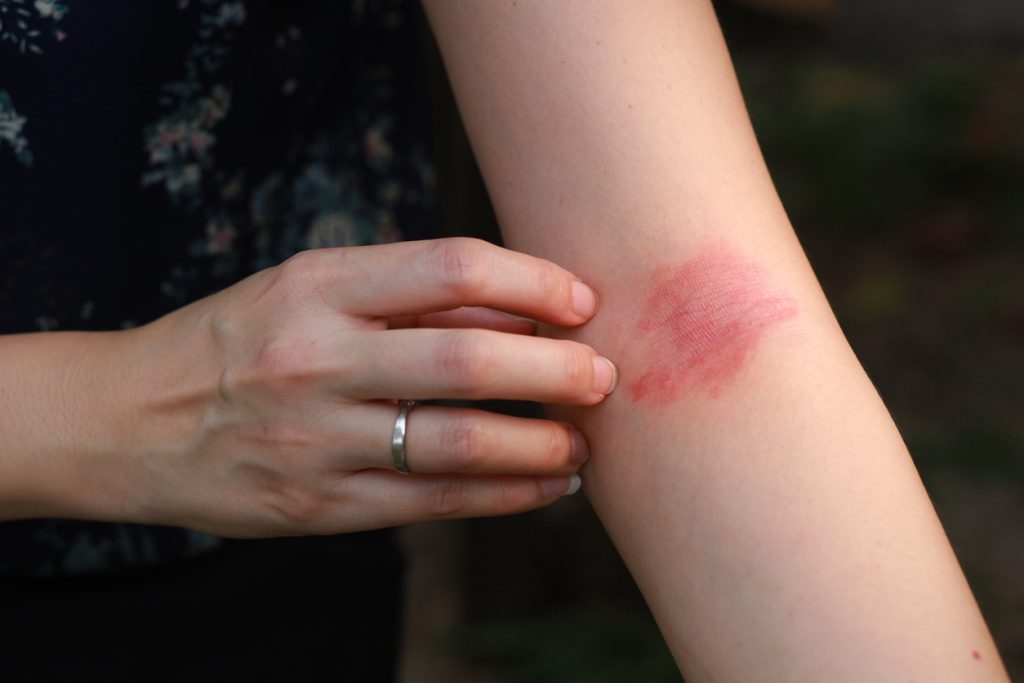
The first patient has been dosed in Alphyn’s CLEAR-AD1 Phase 2b clinical trial of Zabalafin Hydrogel in atopic dermatitis (AD) in Australia.
Zabalafin Hydrogel is a novel, first-in-class complex single-source botanical drug with multiple bioactive compounds that provide multiple mechanisms of action, including anti-pruritic, antibacterial, and anti-inflammatory activity. The therapeutic is derived from the company’s Zabalafin Platform with its Multi-Target Therapeutics.
Alphyn secured clearance from the U.S. Food and Drug Administration of its investigational new drug application for Zabalafin Hydrogel in February and is seeking regulatory approval to begin clinical trials in Europe. The company anticipates initiating clinical trials in the U.S. and in Europe later this year.
CLEAR-AD1 Trial
CLEAR-AD1 will evaluate the safety, efficacy, and tolerability of Zabalafin Hydrogel in patients with mild and moderate AD and directly treat the immuno-inflammatory and bacterial causes of the disease at all stages, from AD onset to infection. The trial is a randomized, double-blinded, vehicle-controlled study enrolling patients at two different stages of AD disease progression – one in which bacteria have contributed to the progression of AD but not yet to the infection stage, and the other in which bacteria have contributed to the progression of AD to the infection stage.
“The initiation of patient dosing in our CLEAR-AD1 Phase 2b clinical trial is another significant milestone for Alphyn, as we strive to bring to market our single comprehensive treatment for patients who suffer from this often-devastating chronic disease,” says Alphyn CEO Neal Koller in a news release. “With the unique properties of our multi-target therapeutic platform, Zabalafin Hydrogel has the potential to overcome the limitations of current therapeutics and to offer patients a safe, effective therapy for worry-free, long-term continuous use.”
Alphyn completed two AD Phase 2a clinical trials that met all primary and secondary endpoints, demonstrating Zabalafin Hydrogel significantly improved pruritus, quality of life, inflammation, as well as control of bacteria-associated and other AD flares, and clearance of AD skin where the bacteria progressed AD to infection stage. In addition to its strong efficacy results, Zabalafin Hydrogel demonstrated excellent safety, side-effect, and patient tolerability.