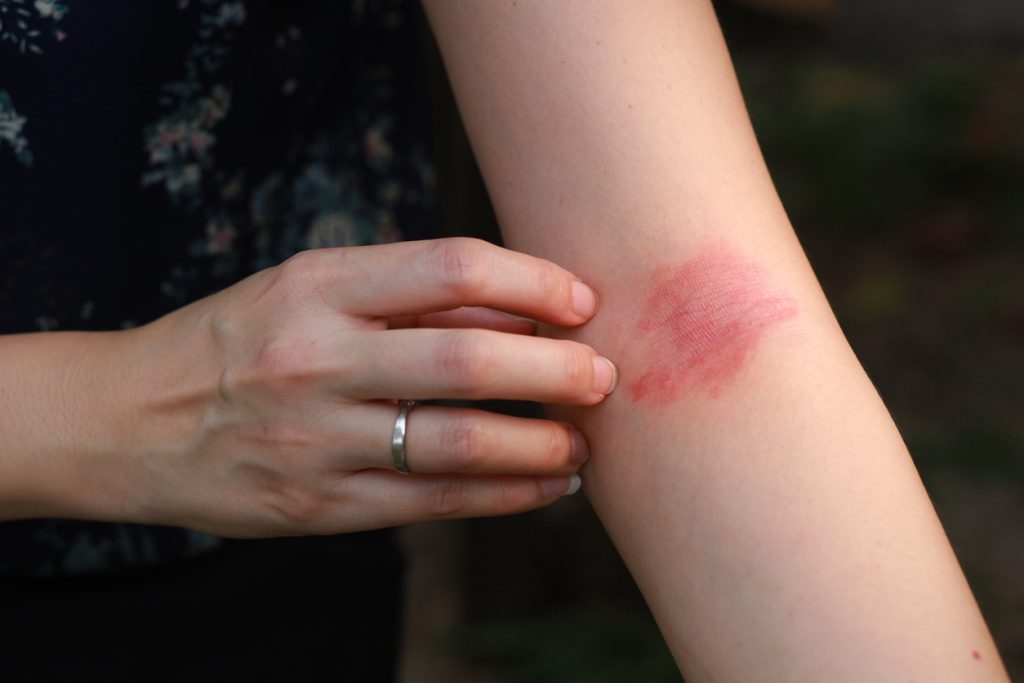
Adult atopic dermatitis (AD) patients previously treated with dupilumab (Dupixent, Regeneron and Sanofi) who received 400mg eblasakimab weekly achieved Eczema Area Severity Index (EASI) 90, and 66.7% achieved a Validated Investigator Global Assessment (vIGA) score of 0 or 1 after 16 weeks compared to 14.3% of patients on placebo, according to positive interim results from a Phase 2 study.
Aslan’s eblasakimab is a potential first-in-class monoclonal antibody targeting the IL-13 receptor subunit of the Type 2 receptor.
The primary endpoint, which is the percent change in EASI score from baseline to week 16, was statistically significant when compared to placebo, even though the interim analysis was not powered for statistical significance due to the sample size. Fully 73.3% (11/15) of eblasakimab-treated patients achieved a reduction in EASI score of at least 75% from baseline (EASI-75) compared to 14.3% (1/7) on placebo.
Treatment was well-tolerated and no new safety signals were identified. There were no reports of conjunctivitis or injection site reactions in the active or placebo arm.
“Most patients on eblasakimab achieved EASI-90 and vIGA of 0 or 1 after just 16 weeks of treatment, with numbers unprecedented in other biologics AD studies. Notably, in patients that previously had an inadequate response to dupilumab, two-thirds achieved EASI-90 and vIGA 0 or 1 when treated with eblasakimab,” says Dr Carl Firth, Chief Executive Officer of Aslan Pharmaceuticals, in a news release.
The interim data will be submitted for presentation at an upcoming scientific conference. Aslan plants to announce the topline readout from the full dataset of the TREK-DX study at the end of this year and to optimize the dose regimen for patients in the planned Phase 3 studies of eblasakimab.