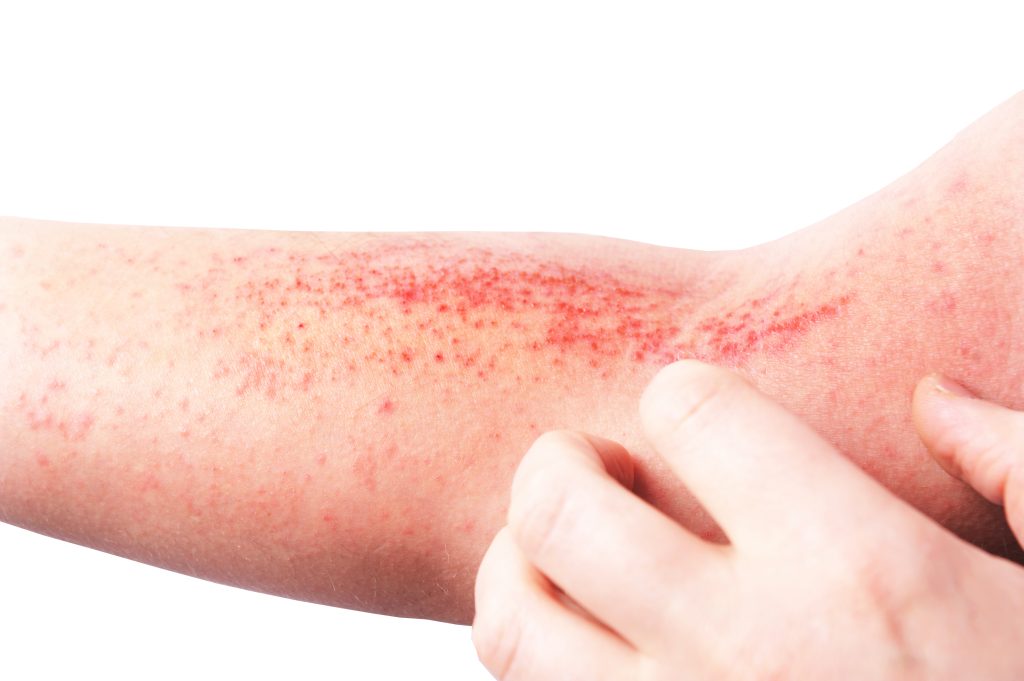
LEO Pharma’s temtokibart, an investigational interleukin (IL)-22RA1 antagonist, performed well in adults with moderate-to-severe atopic dermatitis (AD), according to topline results from a Phase 2b trial.
Temtokibart is an investigational monoclonal antibody for the treatment of moderate-to-severe AD, which blocks the IL-22RA1 receptor subunit, thereby inhibiting the effect of the IL-22 cytokine – known to be elevated in patients with atopic dermatitis.
Evaluate Safety of Different Doses
The study was a randomized, double-blind, placebo-controlled, multi-site, parallel-group, dose-finding trial to evaluate the efficacy and safety of different doses of subcutaneously administered temtokibart, also called LEO 138559, in adults with moderate-to-severe AD.
The trial achieved positive results for the primary endpoint based on percentage change in EASI (Eczema Area and Severity Index) from baseline to Week 16 for three highest doses in adults with moderate-to-severe AD. The treatment was generally well-tolerated, with no dose dependency, and the majority of adverse events observed were non-serious, mild, or moderate in severity, and not considered treatment related.
LEO Pharma is currently collecting and evaluating the full dataset.
Results to Be Presented Later
Detailed results from the phase 2b trial are planned to be submitted for scientific presentation and publication at a later date.
LEO Pharma and argenx formed a strategic alliance in 2015 to develop antibody-based solutions for the treatment of chronic inflammation that underlies many skin conditions. LEO Pharma and argenx jointly developed temtokibart under this research agreement, and LEO Pharma has subsequently obtained the exclusive license to develop and commercialize temtokibart.