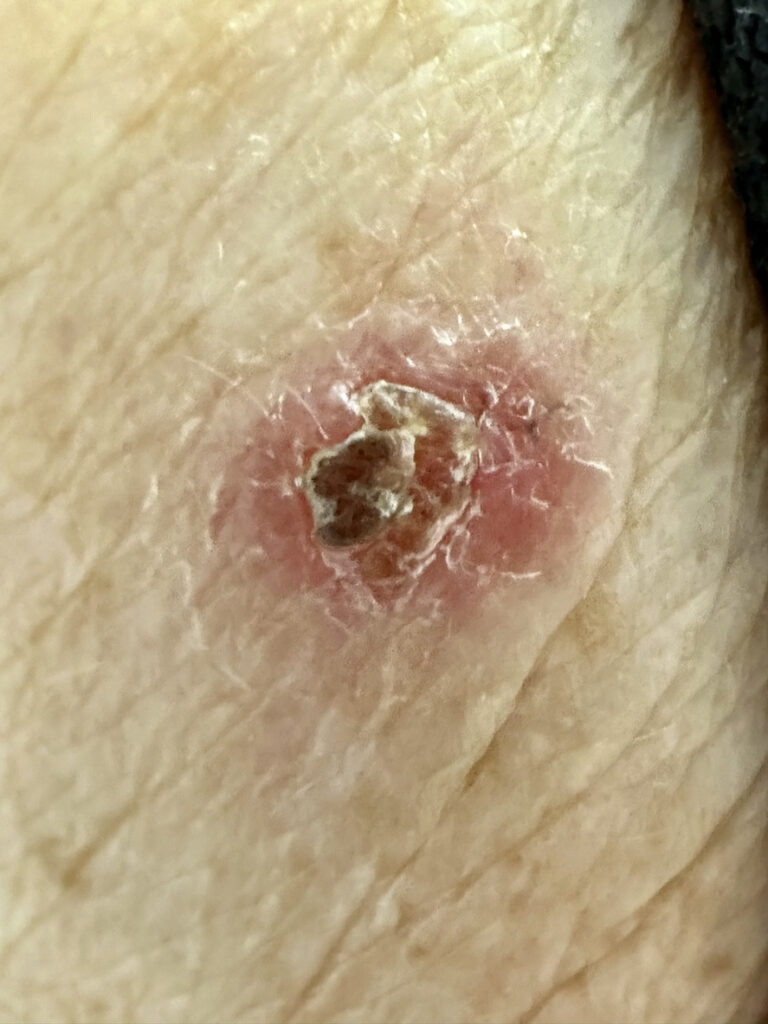
VP-315 (ruxotemitide), a novel oncolytic peptide immunotherapy, continues to show promise for the treatment of basal cell carcinoma (BCC), according to new Phase 2 study results presented at the Society for Immunotherapy of Cancer 40th Annual Meeting in National Harbor, MD.
In Part 2 of the Phase 2 study, 82 subjects (92 tumors) received once-daily injections of 8mg of Verrica’s VP-315 for two to three consecutive days in up to two target lesions. The exploratory analysis evaluated immune responses to VP-315 treatment in the tumor microenvironment (TME) in a subset of 22 subjects (24 tumors) from Part 2 of a Phase 2 multi-center, proof-of-concept study (NCT05188729).
VP-315 was well-tolerated, with no treatment-related serious adverse events, the study showed.
All patients experienced a reduction in tumor size, including:
- 51% complete histologic clearance rate
- 71% reduction in tumor size among patients with residual carcinomas
- 86% overall tumor-size reduction
- 97% objective response rate (post-hoc analysis)
Moreover, histologic assessment in non-injected lesions suggests a potential abscopal-like effect.
Multiplex immunofluorescence immunohistochemistry was performed on paired tumor biopsies collected before treatment and 12–14 weeks after treatment. These results demonstrated that VP-315 reprograms the TME toward a more immunologically active and anti-tumor phenotype, as evidenced by:
- Significant increases in the densities of CD3+, CD3+/CD4+ helper, and CD3+/CD8+ cytotoxic T cells
- Increased B-cell (CD20+) infiltration in the tumor region, suggesting activation of humoral immunity
- Reduction in immunosuppressive cell populations
Collectively, these findings indicate that VP-315 treatment reduces immunosuppression and enhances immune activation within and around the tumor. The observed immunologic cell changes support a mechanistic rationale for abscopal-like immune effects observed in non-treated lesions.
“We are particularly encouraged by the immunologic profile we’re seeing with VP-315, which supports our view that this local, short-term therapy not only destroys tumor cells directly but also stimulates a potent local immune response,” says Jayson Rieger, PhD, MBA, President and Chief Executive Officer of Verrica Pharmaceuticals, in a news release. “These data help explain the mechanism for the clinical safety and efficacy data previously reported and support the development of VP-315 as a potential first-in-class, non-surgical immunotherapy option for patients with BCC and further reinforces our confidence in VP-315’s ability to address a significant unmet need in dermatologic oncology.”
Rieger continues, “With these data and the clarity and broad agreement we received in our End-of-Phase 2 meeting with the FDA on advancing this unique and promising therapy into a pivotal Phase 3 program, we are continuing our preparatory activities and our exploration of new opportunities to fund the BCC program, which may include strategic, non-dilutive partnerships for both the development as well as post-approval commercialization for this potential multi-billion-dollar opportunity in the most common form of skin cancer.”
The new data was presented as a poster and during an oral session at the meeting.