By Linda Cox, MD
In my years of practicing aesthetic medicine, I’ve seen the field evolve rapidly. Patients today are looking for treatments that are not only effective but also fast-acting, natural-looking, and long-lasting. When I discovered the promising Phase 3 data supporting relabotulinumtoxinA’s treatment of glabellar lines and lateral canthal lines, I saw an opportunity to offer something truly innovative to my patients in Germany.
Galderma’s relabotulinumtoxinA is approved as Relfydess in regions including Australia and Europe. It is indicated for the temporary improvement in the appearance of moderate-to-severe glabellar lines at maximum frown and lateral canthal lines seen at maximum smile, alone or in combination, in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient. RelabotulinumtoxinA is not currently approved by the U.S. Food & Drug Administration (FDA) for any use.
The ready-to-use liquid formulation of relabotulinumtoxinA stood out to me as a potential game-changer for both clinical efficiency and patient outcomes. Since incorporating it into my practice, I’ve seen these benefits translate into real-world improvements. Not having to reconstitute the product has made a noticeable difference; it saves time, reduces the risk of dosing errors, and ensures consistency in dosing. This is especially important in a clinic like mine, where multiple prac titioners work under one roof. The formulation’s simplicity has also made scheduling more flexible and allowed me to spend more time on consultations and tailoring treatments, ultimately improving the overall patient experience.
These advantages stem from the Precipitation-free Extraction and Activity-preserving Refined Liquid (PEARL) Technology behind the molecule’s formulation. This multi-step purification method eliminates the need for freeze-drying and reconstitution, keeping the core molecule in a stable liquid state throughout. It removes complexing proteins and impurities while preserving the molecule’s natural form and potency. The result is a highly active, complex-free formulation that enables simple volumetric dosing and supports consistent, reliable outcomes.
Beyond the consistency of results, there’s the speed of onset. Many of my patients begin to see an effect after just one day after treatment, which is rare in this category, and aligns with findings from the READY-1 and READY-2 trials, where 39% of patients saw improvements in glabellar lines and 34% in lateral canthal lines from day one.1,2 By the first month, up to 96% of patients reported satisfaction with their results.1,2 One of my patients described the rapid onset of results as feeling very pleasant and natural, saying she didn’t feel like she was “wearing a mask,” which patients sometimes experience with other treatments. Another patient said she appreciated the natural appearance of the results and that she would consider the treatment again even though she didn’t experience a faster onset in her case. These individual experiences reinforce what I’ve seen more broadly: Patients value the speed of onset and the subtlety of the effect.
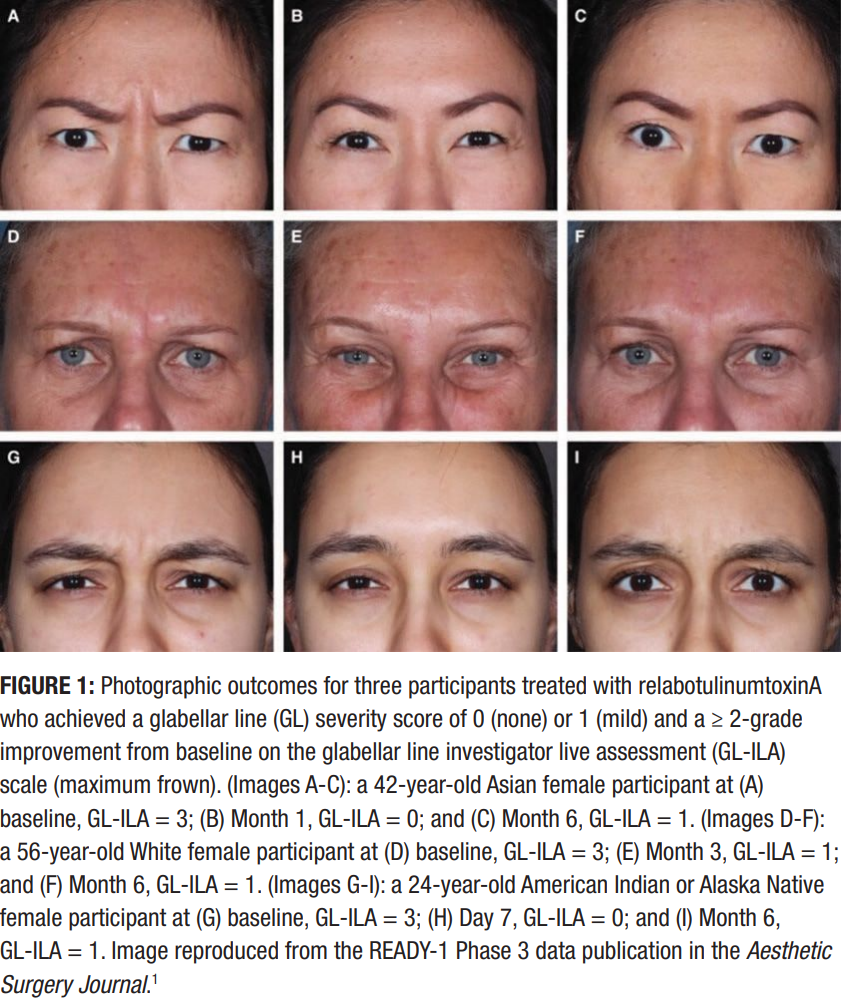
Having seen the clinical data that up to 75% of patients maintained improvements for six months with relabotulinumtoxinA (see Figures 1 and 2),1,2 I was also pleased to observe this longevity of results in my own practice. The duration of the effect is something my experienced patients especially appreciate as it means fewer appointments and longer-lasting outcomes. Many of them comment on the natural, refreshed look they achieve, which I attribute in part to the formulation’s underlying technology. My patients also value the convenience of needing fewer treatments, with some describing it as a real time-saver.
 |
 |
In terms of safety, my clinical experience aligns well with the Phase 3 data. The most commonly reported side effects are mild and transient and typically include headache or discomfort at the injection site.1,2 I’ve observed that these side effects can be mitigated by ensuring the product is not too cold at the time of administration. That said, some of my patients report no side effects at all, which further supports the product’s tolerability in everyday practice.
Just as the safety profile has been reassuring, the level of patient satisfaction has also stood out in my experience with relabotulinumtoxinA. In the RELAX Phase 3b trial, more than half of patients treated with relabotulinumtoxinA reported increased confidence and felt they looked great for their age, even up to 12 months.3 I’ve seen this reflected in my own patients, some of whom describe feeling more like themselves again, and more confident in social and professional settings. One patient told me the treatment gave her a mental boost and an “aesthetic glow-up,” highlighting her features in a way that made her feel “naturally beautiful.” These kinds of responses speak to the deeper value patients place on the subtlety of the treatment.
In today’s social media landscape, many celebrities attract attention by enhancing their appearance in subtle, natural ways that seemingly reverse the aging process. These transformations often prompt patient inquiries in my clinic and highlight the growing demand for treatments that are not only effective but also sustainable and long-lasting. In their search for the fountain of youth, my patients aren’t just looking for quick fixes. They want treatments that support their confidence and well-being over time, and I’m glad to offer options that meet those expectations.
When patients are happy with their results, they return not just for neuromodulation, but for other treatments, too. The doctor-patient relationship is a journey, and offering advanced, effective solutions fosters loyalty and makes for a more fulfilling practice.
References
- Shridharani SM, Moradi A, Donofrio L, et al. Efficacy and safety of relabotulinumtoxinA, a new ready-to-use liquid formulation botulinum toxin: Results from the READY-1 double-blind, randomized, placebo-controlled phase 3 trial in glabellar lines. Aesthet Surg J. 2024;44(12):1330–1340. https://pmc.ncbi.nlm.nih.gov/articles/PMC11566037/
- Ablon G, Bank D, Kontis TC, et al. Efficacy and safety of relabotulinumtoxinA liquid botulinum toxin in the treatment of lateral canthal lines: Results from the phase 3 READY-2 study. Dermatol Surg. 2025;51(3):277–283. https://pubmed.ncbi.nlm.nih.gov/39692332/
- Moradi A, Schlessinger J, Kaufman-Janette J, et al. Aesthetic improvement and subject satisfaction with liquid relabotulinumtoxinA treatment of glabellar lines in a 12-month, randomized, controlled trial. E-poster 145498 presented at the International Master Course on Aging Science (IMCAS) 2025 World Congress. https://www.imcas.com/en/attend/imcas-world-congress-2025/program/session/56199
DISCLOSURES
Linda Cox, MD, reports no relevant financial disclosures.
Copyright Disclosure
Images are reproduced for educational purposes from publicly available articles in the Aesthetic Surgery Journal and Dermatological Surgery. Images are the copyright of the authors of the respective journal articles. Figure 1 was originally published by Oxford University Press on behalf of The Aesthetic Society. Figure 2 was originally published by Wolters Kluwer Health, Inc. on behalf of the American Society for Dermatologic Surgery, Inc.

