Several new therapies are coming down the pike for the treatment of lichen planus, a chronic inflammatory condition that affects an estimated 1% to 2% of the global population, according to a new report from Research and Markets.
There are currently no drugs specifically approved for lichen planus and available treatments are primarily palliative.
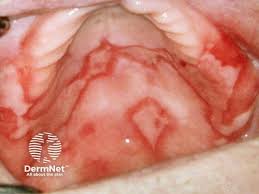
LP-310, developed by Lipella Pharmaceuticals for the treatment of oral lichen planus (OLP), is currently in Phase 2 clinical trials. Similarly, Incyte and AFYX Therapeutics are developing new therapeutics for lichen planus.
LP-310 is an oral rinse formulation. Lipella has received an Investigational New Drug (IND) approval for a Phase 2a multicenter dose-escalation trial evaluating the safety and efficacy of LP-10 in subjects with symptomatic OLP.
Incyte is investigating the efficacy and safety of ruxolitinib cream in cutaneous lichen planus. Ruxolitinib cream is a topical Janus kinase (JAK)1/JAK2 inhibitor. Proof of concept was previously demonstrated in a single-arm, open-label trial of 12 patients with cutaneous lichen planus
AFYX Therapeutics is developing rivelin-CLO, a patch that attaches to mucosal surfaces to deliver medication. In a phase IIb trial, Rivelin-CLO met primary and secondary endpoints in OLP. A study found that the Rivelin-CLO patch was more effective than a placebo in improving OLP symptoms. AFYX-002 is a topical drug designed to treat mucosal disorders, primarily OLP, and AFYX-003 also targets OLP and other mucosal diseases.
Stay tuned.
PHOTO CREDIT: DermNet