Neffy, a needle-free epinephrine nasal spray, showed statistically significant and clinically meaningful improvement in pruritus, hives, body surface area and erythema in treatment-resistant chronic spontaneous urticaria (CSU) patients, according to phase 2 data presented at the 2024 American Academy of Allergy, Asthma and Immunology (AAAAI) in Washington, D.C.
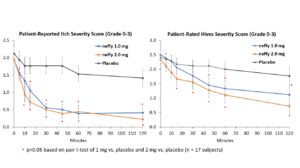
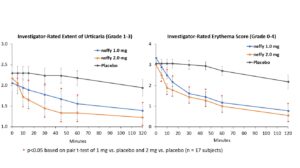
The trial met its primary endpoints with both 1 mg and 2 mg neffy demonstrating statistically significant and clinically meaningful changes from baseline in itch, hives, urticaria and erythema scores as early as five minutes after dosing.
“The Phase 2 results in this highly refractory patient population are impressive and encouraging as they indicate neffy could be a game-changing therapeutic advance in the treatment of urticaria,” says David Bernstein, MD, Emeritus Professor of Pediatrics at the Cincinnati Children’s Hospital Medical Center, a former member of the Joint Task Force on Practice Parameters (urticaria guidelines) and Principal Investigator of the Study, in a news release. “Urticaria symptoms clear within minutes after dosing, and neffy could offer patients a novel treatment option to address existing gaps in the efficacy of currently available antihistamines or biologics.”
The EPI-U01 study was a randomized, placebo-controlled, cross-over study evaluating the safety and efficacy of epinephrine nasal spray in patients with chronic spontaneous urticaria (CSU) treated with chronic medications who were still experiencing flares. This oral presentation includes data analysis from 18 adult patients as of the cut-off date who enrolled in this study and returned to the clinical site while experiencing a flare, where they were randomized to receive either a single treatment of 1 mg or 2 mg neffy or placebo in a crossover design.
All subjects had flares with pruritus and hives scores greater than or equal to 2 on a 3-point severity scale, despite all patients having been treated with antihistamines or Xolair.
There was no meaningful difference in efficacy on patient-reported itch severity score, patient-reported hives severity score, investigator-related extent of urticaria, or investigator-related erythema score between 1 mg and 2 mg neffy doses, indicating that the 1 mg dose may be sufficient to activate the beta-2 adrenergic receptors responsible for stopping the mast cell degranulation and allergic mediator release that leads to an urticaria flare.
Neffy was well-tolerated, with adverse events reported in 8 subjects, which were all mild or moderate in severity. The most common adverse event reported was nasal discomfort in 5 subjects. There were no serious adverse events.
ARS Pharma plans to initiate a placebo-controlled outpatient urticaria study in patients treated with antihistamines who experience frequent acute flares later in 2024 followed by the potential initiation of a single pivotal efficacy study in 2025. This would follow the anticipated FDA approval of neffy for allergic reactions (Type I) including anaphylaxis in the the second half of 2024.
The U.S. Food and Drug Administration (FDA) opted not to approve neffy for emergency treatments of severe allergic reactions in September 2023. Approval of Neffy nasal spray was widely anticipated by the allergy community. An FDA advisory panel voted to recommend approval of the drug for children and adults in May. The FDA is not obligated to follow the advice of their advisory panels, but they usually do. Instead, the FDA told the drug’s maker ARS Pharmaceuticals that it needed to conduct another study on the drug before it is approved, the company said in a statement.


