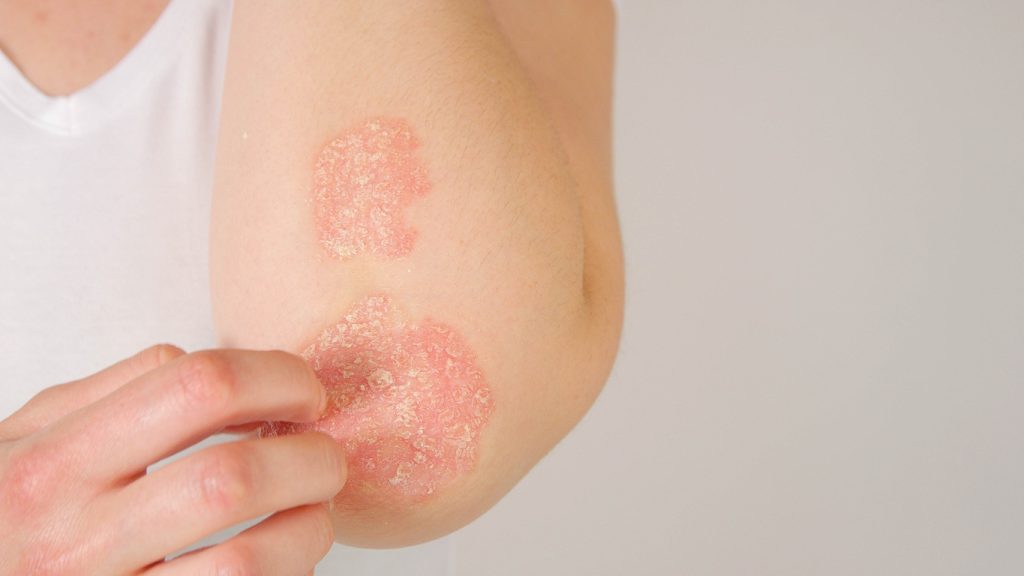
The first patient has been dosed in the Artax’s Phase 2a trial evaluating the safety and biomarker responses of AX-158 in a first proof of mechanism trial in psoriasis.
AX-158 has potential to be the first immunomodulator in the Nck blocker class, the Company states.
Safety and efficacy results are expected in second half of 2024.
“The new Nck blocker class of agents, AX-158, has the potential to change how we treat autoimmune disease through targeting and tempering TCR responses so the body only, and properly, reacts against strong pathogens.”
James G. Krueger, MD, Ph.D, Head of the Laboratory for Investigative Dermatology at The Rockefeller University in New York City and Artax Scientific Advisory Board member.
By selectively targeting Nck function, which plays a critical role in immune system function, AX-158 recalibrates the body’s T-cell receptor (TCR) responses. This recalibration to responses allows the immune system to continue functioning properly and activate only when it recognizes true disease threats – preventing self-activation without causing immune suppression that makes the body susceptible to numerous infections.
“The new Nck blocker class of agents, AX-158, has the potential to change how we treat autoimmune disease through targeting and tempering TCR responses so the body only, and properly, reacts against strong pathogens,” says James G. Krueger, MD, PhD, Head of the Laboratory for Investigative Dermatology at The Rockefeller University in New York City and Artax Scientific Advisory Board member. “I am excited to see how this new mechanism of action translates into biomarker changes and patient responses, where to date only targeted immunosuppression has been applied.”
For Phase 2a study inquiries, contact: ax-158-1011@artaxbiopharma.com