By Sawyeh Maher, BS, and Peter A. Lio, MD
- Introduction
- Barrier Protection and Moisturizer Therapy
- Paraffin Bath
- Digital Self-monitoring and Education via Smartphone App
- Phototherapy
- Conclusion
Introduction
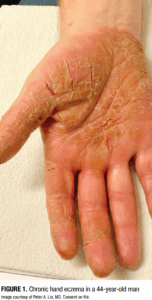 Chronic hand eczema (CHE) is a common and debilitating condition characterized by eczematous lesions on the hands and wrists that persist for longer than three months or recur at least twice per year (Figure 1). CHE is commonly associated with pain and itch and often carries a social and economic burden.1,2,3,4 Lifetime prevalence is estimated to be about 15%, with a higher incidence in women and young adults.5 CHE is complex and multifactorial, encompassing multiple overlapping subtypes including atopic dermatitis (AD)/atopic hand eczema (AHE), irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and protein contact dermatitis/contact urticaria (PCD/CU). The condition is driven by genetic, immunologic, environmental, and occupational factors that often complicate diagnosis and management.6
Chronic hand eczema (CHE) is a common and debilitating condition characterized by eczematous lesions on the hands and wrists that persist for longer than three months or recur at least twice per year (Figure 1). CHE is commonly associated with pain and itch and often carries a social and economic burden.1,2,3,4 Lifetime prevalence is estimated to be about 15%, with a higher incidence in women and young adults.5 CHE is complex and multifactorial, encompassing multiple overlapping subtypes including atopic dermatitis (AD)/atopic hand eczema (AHE), irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and protein contact dermatitis/contact urticaria (PCD/CU). The condition is driven by genetic, immunologic, environmental, and occupational factors that often complicate diagnosis and management.6
Current CHE therapies range from supportive to systemic interventions. First-line management emphasizes supportive measures such as skin protection, moisturizers, and avoidance of allergens and irritants.7,8 Topical corticosteroids (TCS) and calcineurin inhibitors (TCIs) are common second-line options, though both are limited by side effects, adherence challenges, and reduced efficacy in severe disease.9,10,7,11,12 For refractory disease, systemic agents such as alitretinoin, cyclosporine, or methotrexate may be considered, though they carry the risk of potential toxicity and lack of US approval in some cases.13,14,15 More recently, therapies have emerged that more directly target the pathogenesis of CHE, including biologics and Janus kinase (JAK) inhibitors. Delgocitinib (Anzupgo, LEO Pharma), a pan-JAK inhibitor that was recently approved for CHE, is notable for its effects on the pathways relevant to CHE. Its broad mechanism makes it particularly suitable for CHE with mixed subtypes and allergic triggers, since in such cases there may be multiple immune pathways that are dysregulated.16 While it does not carry a formal boxed warning like other JAK inhibitors, adverse effects such as application-site pain, paresthesia, and bacterial skin infections have been reported.17,18
Despite these advances, no single therapy has fully addressed the multifactorial drivers of CHE. For this reason, non-pharmacologic and lifestyle-based approaches may offer viable adjunctive strategies for CHE.
In this narrative review, we highlight non-pharmacologic and lifestyle-based interventions available for CHE with the strongest evidence, safety, and practicality for clinical use. Priority was given to randomized controlled trials (RCTs), systematic reviews, and large observational studies. Study quality was appraised using established tools, including the Jadad scale for randomized trials, with complementary reference to Risk of Bias 2.0 (RoB 2), Newcastle–Ottawa, Joanna Briggs Institute (JBI), and A MeaSurement Tool to Assess Systematic Reviews (AMSTAR 2), where appropriate. All adjunct therapies reviewed also presented little to no side effects for participants. Outcomes of interest included Hand Eczema Severity Index (HECSI) and HECSI-R, Investigator’s Global Assessment for CHE (IGA-CHE), itch/pain, quality of life, flare frequency, work-related impact, and adverse events (Table).
Barrier Protection and Moisturizer Therapy
 Barrier protection and moisturizer use are foundational components of CHE management, as most subtypes involve skin barrier dysfunction and increased transepidermal water loss.19,20 Regular use of moisturizers can restore barrier integrity, reduce water loss, and alleviate symptoms such as dryness and pruritus.21 Avoidance of common irritants such as harsh and/or scented soaps, detergents, and work hazards is equally vital for barrier protection.22 Glove use should also be implemented during wet-work and exposures to occupational hazards, with a focus on limiting occlusion and irritation.23 Barrier protectants such as dimethicone-based creams can reduce irritant-induced flares, particularly when combined with use of gloves and moisturizers.24,25
Barrier protection and moisturizer use are foundational components of CHE management, as most subtypes involve skin barrier dysfunction and increased transepidermal water loss.19,20 Regular use of moisturizers can restore barrier integrity, reduce water loss, and alleviate symptoms such as dryness and pruritus.21 Avoidance of common irritants such as harsh and/or scented soaps, detergents, and work hazards is equally vital for barrier protection.22 Glove use should also be implemented during wet-work and exposures to occupational hazards, with a focus on limiting occlusion and irritation.23 Barrier protectants such as dimethicone-based creams can reduce irritant-induced flares, particularly when combined with use of gloves and moisturizers.24,25
The evidence supports the importance of barrier strength in managing CHE. In a randomized, open-label study of 53 patients with resolved hand eczema, use of a 5% urea-containing barrier-strengthening moisturizer significantly delayed disease relapse compared to no treatment, with median relapse-free intervals of 20 days vs. 2 days (P = 0.04).26 Another RCT of 102 CHE patients found that a ceramide-containing cream significantly improved HECSI scores at three months compared with usual care (odds ratio 2.6; 95% CI 1.3–5.2).27
Beyond product use, patient education about barrier protection improves adherence and outcomes. A prospective study of 71 patients found that integrating therapeutic patient education into routine CHE care significantly improved barrier-protective behaviors—including moisturizing, glove use, and adherence to avoidance strategies—and was associated with improvements in clinical severity (the modified Total Lesion Symptom Score, or mTLSS), quality of life (The Dermatology Life Quality Index, or DLQI), and work productivity (the Work Productivity and Activity Impairment, or WPAI) at three months.28 No serious adverse events were reported across any of these studies.
These findings align with a Cochrane Database Systematic Review of 77 trials and observational studies, which demonstrated that regular moisturizer and barrier-supportive cream use prolongs time to flare, reduces flare frequency, and lowers corticosteroid requirements.29
Paraffin Bath
 Paraffin wax baths have been explored as an adjunct treatment for CHE. Paraffin, a petroleum-derived hydrocarbon, is commonly used as an emollient in skincare products and cosmetics. Its low melting point allows for submersion that provides superficial heat, improves blood flow, enhances the skin barrier, and reduces discomfort.30,31 In an RCT of 60 patients with moderate-tosevere CHE, twice-daily paran baths for 12 weeks reduced SCORing Atopic Dermatitis (SCORAD) scores by 28.6% compared to 0.41% in controls. Reported symptoms, including itch and sleep quality, improved by 47% vs. 5.5% in controls, while DLQI improved by 60% vs. 3.8% in controls. No adverse events were reported.32 A smaller prospective study of 13 patients with CHE similarly found high patient satisfaction and symptomatic improvement without adverse effects.31
Paraffin wax baths have been explored as an adjunct treatment for CHE. Paraffin, a petroleum-derived hydrocarbon, is commonly used as an emollient in skincare products and cosmetics. Its low melting point allows for submersion that provides superficial heat, improves blood flow, enhances the skin barrier, and reduces discomfort.30,31 In an RCT of 60 patients with moderate-tosevere CHE, twice-daily paran baths for 12 weeks reduced SCORing Atopic Dermatitis (SCORAD) scores by 28.6% compared to 0.41% in controls. Reported symptoms, including itch and sleep quality, improved by 47% vs. 5.5% in controls, while DLQI improved by 60% vs. 3.8% in controls. No adverse events were reported.32 A smaller prospective study of 13 patients with CHE similarly found high patient satisfaction and symptomatic improvement without adverse effects.31
Digital Self-monitoring and Education via Smartphone App
 Digital health tools in dermatology have emerged as a powerful avenue for patients to connect with their providers and optimize treatment outcomes. In CHE, smartphone applications provide real-time monitoring and education, which may improve adherence, outcomes, and patient–provider communication.33
Digital health tools in dermatology have emerged as a powerful avenue for patients to connect with their providers and optimize treatment outcomes. In CHE, smartphone applications provide real-time monitoring and education, which may improve adherence, outcomes, and patient–provider communication.33
An RCT of 87 patients with chronic hand and foot eczema compared frequent in-person visits plus access to an educational app with in-person visits alone. The app allowed the ability to upload photos, track symptoms, and send messages to the dermatologist. Patients in the intervention group displayed significant reduction in HECSI scores over 60 weeks (coecient of −1.108, P ≤ 0.001). Benefits were dose-dependent, with high-frequency users with ≥20% app engagement showing additional improvements in quality of life (DLQI) and symptom control. The app also reliably assessed disease severity, with patient-submitted photos strongly correlating with physician-scored HECSI (P = 0.885, P < 0.001). No adverse events were reported.34
Phototherapy
 Phototherapy has been a frequently employed treatment for refractory eczema. Various modalities exist—including psoralen plus ultraviolet A (PUVA), UVA1, broadband UVB, and narrowband UVB (NB–UVB)—with NB–UVB emerging as the most used due to its favorable balance of efficacy, safety, and practicality.35
Phototherapy has been a frequently employed treatment for refractory eczema. Various modalities exist—including psoralen plus ultraviolet A (PUVA), UVA1, broadband UVB, and narrowband UVB (NB–UVB)—with NB–UVB emerging as the most used due to its favorable balance of efficacy, safety, and practicality.35
In a randomized pilot study, CHE patients were assigned to receive either NB–UVB or PUVA twice weekly for 12 weeks. At Week 12, 23% of NB–UVB patients achieved a “Clear” or “Almost Clear” Physician’s Global Assessment response, with comparable improvements in the mTLSS and DLQI. Adverse events were minimal.36
A Cochrane systematic review further supports NB–UVB for use in atopic-driven eczema, and found that patients show significant symptomatic improvement after 12 weeks.37 The American Academy of Allergy, Asthma, and Immunology also highlights NB–UVB as effective for atopic eczema, with improvements displayed in both severity and itch. No adverse effects or increased risk of skin cancer were discovered.38
Conclusion
CHE remains a challenging and often refractory condition, with widely used conventional therapies limited by side effects, adherence challenges, and variable efficacy across subtypes. While traditional pharmacologic options are expanding, non-pharmacologic and lifestyle-based strategies—including barrier optimization, paraffin baths, digital self-monitoring tools, and phototherapy—remain safe, low-risk, and practical adjunct therapies that can enhance outcomes and patient quality of life. While the data is promising, further investigation is warranted to better define the role of these methods as adjunctive or stand-alone therapies for CHE.
REFERENCES
- Weidinger S, Novak N. Hand eczema. Lancet. 2024;404(10470):2476–2486. https://pubmed.ncbi.nlm.nih.gov/39615508/
- Sloot MM, Loman L, Romeijn GLE, Rosenberg FM, Arents BWM, Schuttelaar MLA. Patients’ perspectives on quality of care for chronic hand eczema: A qualitative study. Contact Dermatitis. 2022;86(3):204–212. https://pubmed.ncbi.nlm.nih.gov/34871458/
- Zalewski A, Krajewski PK, Szepietowski JC. Psychosocial consequences of hand eczema—A prospective cross-sectional study. J Clin Med. 2023;12(17):5741. https://pubmed.ncbi.nlm.nih.gov/37685808/
- Armstrong A, Hahn-Pedersen J, Bartlett C, Glanville J, Thyssen JP. Economic burden of chronic hand eczema: A review. Am J Clin Dermatol. 2022;23(3):287–300. https://pubmed.ncbi.nlm.nih.gov/35258783/
- Quaade AS, Simonsen AB, Halling AS, Thyssen JP, Johansen JD. Prevalence, incidence, and severity of hand eczema in the general population – A systematic review and meta-analysis. Contact Dermatitis. 2021;84(6):361–374. https://pubmed.ncbi.nlm.nih.gov/33548072/
- Agner T, Aalto-Korte K, Andersen KE, et al. Classification of hand eczema. J Eur Acad Dermatol Venereol. 2015;29(12):2417–2422. https://doi.org/10.1111/jdv.13308
- Elsner P, Agner T. Hand eczema: treatment. J Eur Acad Dermatol Venereol. 2020;34(S1):13–21. https://doi.org/10.1111/jdv.16062
- Weisshaar E. Chronic hand eczema. Am J Clin Dermatol. 2024;25(6):909–926. https://pubmed.ncbi.nlm.nih.gov/39300011/
- Brookes TSR, Barlow R, Mohandas P, Bewley A. Topical steroid withdrawal: an emerging clinical problem. Clin Exp Dermatol. 2023;48(9):1007–1011. https://pubmed.ncbi.nlm.nih.gov/37119282/
- Pemberton MA, Kimber I. Propylene glycol, skin sensitisation and allergic contact dermatitis: A scientific and regulatory conundrum. Regul Toxicol Pharmacol. 2023;138:105341. https://pubmed.ncbi.nlm.nih.gov/36702195/
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. https://doi.org/10.1111/jdv.18429
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-based recommendations. Ann Allergy Asthma Immunol. 2024;132(3):274–312. https://doi.org/10.1016/j.anai.2023.11.009
- Ho JSS, Molin S. A review of existing and new treatments for the management of hand eczema. J Cutan Med Surg. 2023;27(5):493–503. https://pubmed.ncbi.nlm.nih.gov/37496489/
- Dadlani C, Orlow SJ. Treatment of children and adolescents with methotrexate, cyclosporine, and etanercept: Review of the dermatologic and rheumatologic literature. J Am Acad Dermatol. 2005;52(2):316–340. https://pubmed.ncbi.nlm.nih.gov/15692480/
- Dubin C, Duca ED, Guttman-Yassky E. Drugs for the treatment of chronic hand eczema: Successes and key challenges. Ther Clin Risk Manag. 2020;16:1319–1332. https://pubmed.ncbi.nlm.nih.gov/33408476/
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, longterm extension study. J Am Acad Dermatol. 2020;82(4):823–831. https://pubmed.ncbi.nlm.nih.gov/32029304/
- Gooderham M, Molin S, Bissonnette R, et al. Long-term safety and efficacy of delgocitinib cream for up to 52 weeks in adults with chronic hand eczema: Results of the phase 3 open-label extension DELTA 3 trial following the DELTA 1 and 2 trials. J Am Acad Dermatol. 2025;93(1):95–103. https://pubmed.ncbi.nlm.nih.gov/40081663/
- Nakagawa H, Igarashi A, Saeki H, et al. Safety, efficacy, and pharmacokinetics of delgocitinib ointment in infants with atopic dermatitis: A phase 3, open-label, and long-term study. Allergol Int. 2024;73(1):137–142. https://pubmed.ncbi.nlm.nih.gov/37100717/
- Tauber M, Bérard E, Lourari S, et al. Latent class analysis categorizes chronic hand eczema patients according to skin barrier impairment. J Eur Acad Dermatol Venereol. 2020;34(7):1529–1535. https://pubmed.ncbi.nlm.nih.gov/31736135/
- Blichmann C, Serup J. Hydration studies on scaly hand eczema. Contact Dermatitis. 1987;16(3):155–159. https://pubmed.ncbi.nlm.nih.gov/3581823/
- Elias PM, Sugarman J. Does moisturizing the skin equate with barrier repair therapy. Ann Allergy Asthma Immunol. 2018;121(6):653–656.e2. https://pubmed.ncbi.nlm.nih.gov/30009880/
- Ahmed ZH, Agarwal K, Sarkar R. Hand dermatitis: A comprehensive review with special emphasis on COVID-19 pandemic. Indian J Dermatol. 2021;66(5):508–519. https://pubmed.ncbi.nlm.nih.gov/35068506/
- Yüksel YT, Symanzik C, Christensen MO, et al. Prevalence and incidence of hand eczema in healthcare workers: A systematic review and meta-analysis. Contact Dermatitis. 2024;90(4):331–342. https://pubmed.ncbi.nlm.nih.gov/38186085/
- Diepgen TL. Occupational skin diseases. J Dtsch Dermatol Ges. 2012;10(5):297–315. https://pubmed.ncbi.nlm.nih.gov/22455666/
- Thyssen JP, Schuttelaar MLA, Alfonso JH, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis. 2022;86(5):357–378. https://pubmed.ncbi.nlm.nih.gov/34971008/
- Lodén M, Wirén K, Smerud K, et al. Treatment with a barrier-strengthening moisturizer prevents relapse of hand-eczema. An open, randomized, prospective, parallel group study. Acta Derm Venereol. 2010;90(6):602–606. https://pubmed.ncbi.nlm.nih.gov/21057743/
- Filon FL, Maculan P, Crivellaro MA, Mauro M. Effectiveness of a skin care program with a cream containing ceramide C and a personalized training for secondary prevention of hand contact dermatitis. Dermatitis. 2023;34(2):127–134. https://pubmed.ncbi.nlm.nih.gov/36939821/
- Tauber M, Lourari S, Bérard E, et al. Positive change in hand care habits using therapeutic patient education in chronic hand eczema. Contact Dermatitis. 2020;82(1):10–17. https://pubmed.ncbi.nlm.nih.gov/31461531/
- van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2(2):CD012119. https://pubmed.ncbi.nlm.nih.gov/28166390/
- Chang YW, Hsieh SF, Horng YS, Chen HL, Lee KC, Horng YS. Comparative effectiveness of ultrasound and paraffin therapy in patients with carpal tunnel syndrome: a randomized trial. BMC Musculoskelet Disord. 2014;15:399. https://pubmed.ncbi.nlm.nih.gov/25428566/
- Mir-Bonafé JF, Serra-Baldrich E, Rozas-Muñoz E, Puig L. Paraffin wax baths for the treatment of chronic hand eczema. Actas Dermosifiliogr. 2017;108(3):261–264. https://pubmed.ncbi.nlm.nih.gov/28010873/
- Waked IS, Ibrahim ZM. Beneficial effects of paraffin bath therapy as additional treatment of chronic hand eczema: A randomized, single-blind, active-controlled, parallel-group study. J Altern Complement Med. 2020;26(12):1144–1150. https://pubmed.ncbi.nlm.nih.gov/33196289/
- Weigandt WA, Schardt Y, Bruch A, et al. Impact of an eHealth smartphone app on quality of life and clinical outcome of patients with hand and foot eczema: Prospective randomized controlled intervention study. JMIR MHealth UHealth. 2023;11:e38506. https://pubmed.ncbi.nlm.nih.gov/36881465/
- Bruch A, Weigandt W, Schardt Y, Herr R, Benecke J, Schmieder A. Improving outcomes and quality of life for patients with hand and foot eczema: Randomized study of a patient-centered monitoring app. J Med Internet Res. 2025;27:e52159. https://pubmed.ncbi.nlm.nih.gov/39836950/
- Knöps E, Spuls P, Duijnhoven R, et al. The UPDATE trial (UVB Phototherapy in Dermatology for ATopic Eczema): study protocol for a randomized controlled trial of narrowband UVB with optimal topical therapy versus optimal topical therapy in patients with atopic eczema. Trials. 2024;25(1):482. https://pubmed.ncbi.nlm.nih.gov/39014498/
- Brass D, Fouweather T, Stocken DD, et al. An observer-blinded randomized controlled pilot trial comparing localized immersion psoralen-ultraviolet A with localized narrowband ultraviolet B for the treatment of palmar hand eczema. Br J Dermatol. 2018;179(1):63–71. https://pubmed.ncbi.nlm.nih.gov/29235664/
- Musters AH, Mashayekhi S, Harvey J, et al. Phototherapy for atopic eczema. Cochrane Database Syst Rev. 2021;10(10):CD013870. https://pubmed.ncbi.nlm.nih.gov/34709669/
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132(3):274–312. https://pubmed.ncbi.nlm.nih.gov/38108679/
ABOUT THE AUTHORS
Sawyeh Maher, BS is a third-year medical student at Rosalind Franklin University of Medicine and Science in Chicago, IL.
Peter A. Lio, MD, is a Clinical Assistant Professor of Dermatology and Pediatrics at Northwestern University Feinberg School of Medicine and a partner at Medical Dermatology Associates of Chicago in Chicago, IL.

