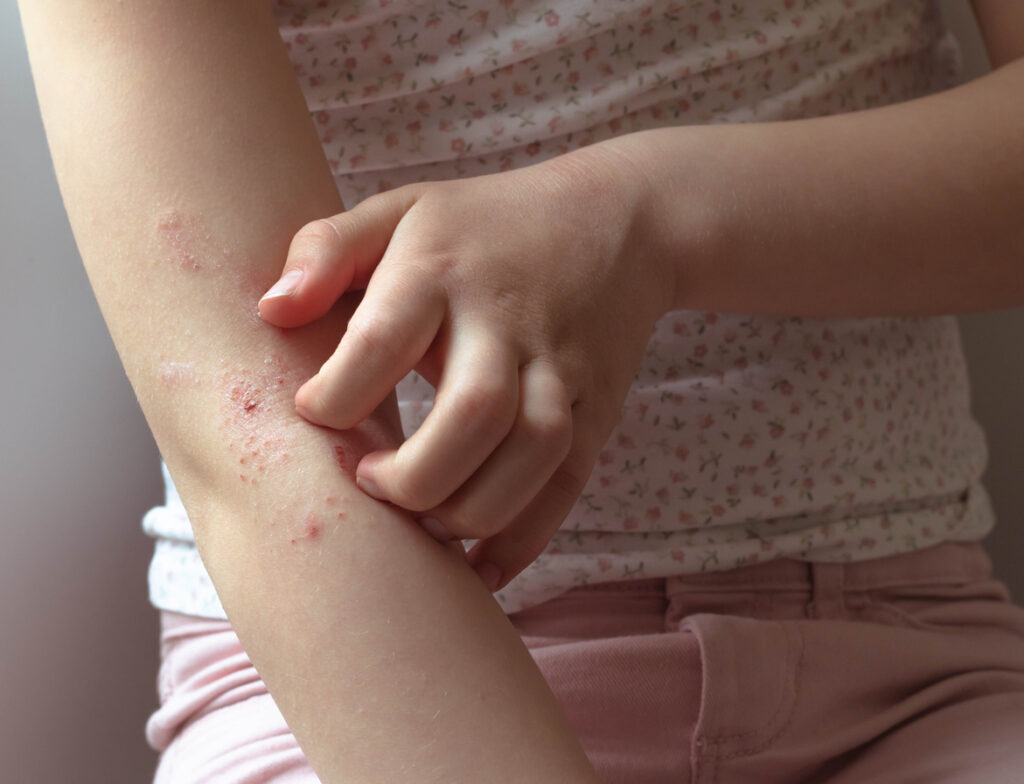
BBT001, Bambusa Therapeutics’ investigational atopic dermatitis (AD) candidate, performed well in the single-ascending dose part of its ongoing Phase I healthy volunteer study.
BBT001 is a first-in-class, multi-targeting, half-life–extended bispecific antibody engineered to block both interleukin (IL)-4Rα and IL-31 signaling. It is currently in Phase 1 clinical development for atopic dermatitis and other Type 2 inflammatory skin diseases.
Study Results
The data demonstrated:
- A favorable safety and tolerability profile across all dose levels with no dose-limiting adverse events.
- A best-in-class pharmacokinetic profile (~33-day half-life), validating Bambusa’s half-life extension technology and supporting flexible dosing regimens.
- Rapid, complete, and sustained IL-4Rα binding and pSTAT6 inhibition observed through Week 8+ following a single dose of BBT001.
- An unprecedented, dose-dependent, rapid, deep, and sustained reduction in thymus and activation-regulated chemokine (TARC) levels after a single dose of BBT001, with potent suppression maintained through Week 8+.
“These results provide the first clinical evidence of BBT001’s potential to become a best-in-disease biologic in atopic dermatitis,” says Thang Ho, PhD, Senior Vice President of Development Sciences at Bambusa Therapeutics, in a news release. “Despite low baseline TARC levels, we observed an unprecedented dose-dependent reduction in healthy volunteers, suggesting synergy between IL-4Rα and IL-31 inhibition. Given the strong correlation between TARC reduction and treatment efficacy in atopic dermatitis, these data give us confidence that BBT001 may deliver faster onset and deeper relief for patients across the Type 2 inflammatory skin disease spectrum.”
The results were presented at the European Academy of Dermatology and Venereology (EADV) 2025 in Paris, France.
First Patient Dosed in Clinical Trial
In related news, the first patient with moderate-to-severe AD has been dosed in a Phase I clinical trial of BBT001.
BT001-001 is a randomized, placebo-controlled, single- and multiple-ascending-dose study in healthy volunteers and adults with moderate-to-severe AD. The trial is evaluating safety, tolerability, pharmacokinetics, immunogenicity, pharmacodynamics, and preliminary clinical activity. Patient enrollment is ongoing, with additional data expected in 2026.
“Achieving patient dosing within 16 months of company inception underscores our pace of execution and represents a powerful moment for Bambusa Therapeutics and the atopic dermatitis community,” adds Shanshan Xu, MD, PhD, Founder & Chief Executive Officer of Bambusa Therapeutics. “With the trial now underway, we feel a deep responsibility to patients to accelerate development, generate proof-of-concept clinical data in patients, challenge, and ultimately redefine the standard of care in atopic dermatitis.”