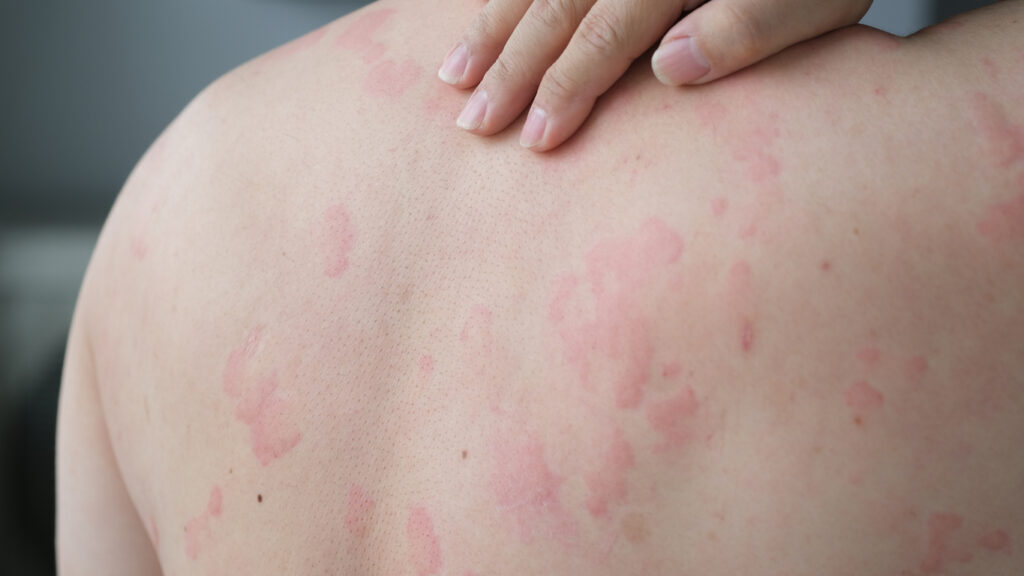
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending the approval of dupilumab (Dupixent, Regeneron & Sanofi) in the European Union (EU) for the treatment of chronic spontaneous urticaria (CSU) in adults and adolescents.
This recommendation covers people aged 12 and up with moderate-to-severe disease, with inadequate response to histamine-1 antihistamines (H1AH) and who are naive to anti-immunoglobulin E (IgE) therapy.
A final decision is expected in the coming months, the companies report.
The positive CHMP opinion is supported by data from two trials in the LIBERTY-CUPID Phase 3 program (Study A and Study C), both of which demonstrated dupilumab significantly reduced itch and hives at 24 weeks compared to placebo. A third trial from the LIBERTY-CUPID program (Study B), conducted in a different CSU patient population, provided additional safety data.
The safety results of the trials were generally consistent with the known safety profile of dupilumab in its approved indications. Adverse events (AEs) more commonly observed with dupilumab than placebo in the trials of adults and adolescents with CSU were injection-site reaction, COVID-19, hypertension, CSU, and accidental overdose.
Dupilumab is approved for CSU in certain adults and adolescents in several countries including Japan and the United States.