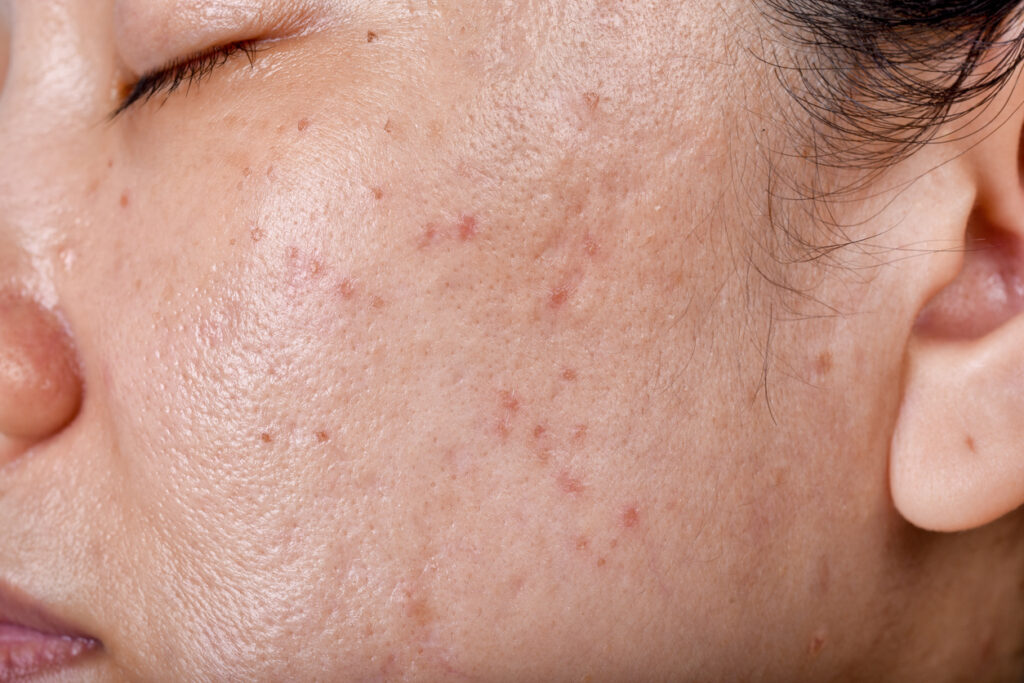
Hoth Therapeutics, Inc. has submitted its Clinical Trial Application (CTA) to the European Medicines Agency (EMA) to expand its ongoing Phase 2 trial of HT-001, a novel topical therapeutic for skin toxicities associated with Epidermal Growth Factor Receptor inhibitors (EGFRi).
Pending EMA review and approval, the Company expects to initiate European patient recruitment in early 2026, complementing active enrollment already underway at multiple U.S. sites.
HT-001 is a once-daily topical gel formulated with U.S. Food and Drug Administration (FDA)-approved neurokinin-1 receptor antagonist (NK1RA). This mechanism mitigates inflammatory pathways triggered by EGFR inhibition, particularly Substance P-driven responses that lead to skin breakdown.
“We are very pleased with the timely CTA submission to the EMA, a pivotal step in advancing our international development of HT-001,” says Robb Knie, Chief Executive Officer of Hoth Therapeutics, in a news release. “Skin toxicities from EGFRi therapies remain an urgent, unmet medical need, and with no FDA- or EMA-approved treatment available, advancing HT-001 in Europe represents a powerful opportunity to improve patient quality of life and drive meaningful value creation for shareholders.”