A drug-induced bullous eruption with a twist

Matthew J. Zirwas, MD, with Cheryl Guttman Krader
CASE HISTORY
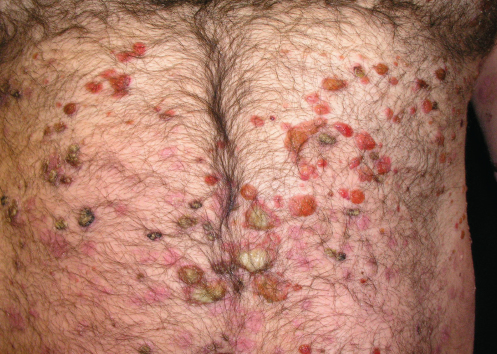
A 40-year-old white man presented with a 2-week history of widespread blisters. Clinical examination showed tense, subepidermal-appearing bullae on mildly erythematous bases (Figure 1). Palms, soles, scalp, and mucous membranes were all spared. On history, the patient reported having ulcerative colitis for which he had been taking a 5-aminosalicylic acid (5-ASA) medication, mesalamine, for at least 5 years. He was not on any other medications and reported that he felt generally well.
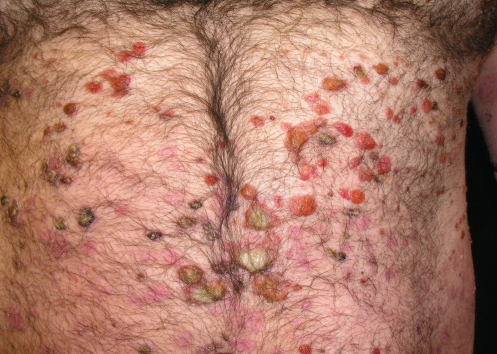 | 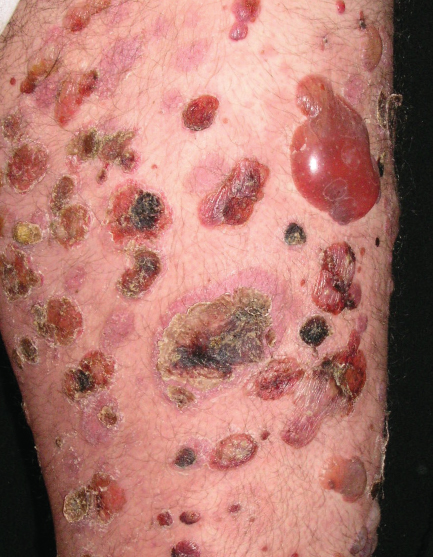 | 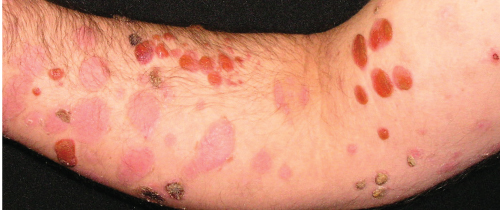 |
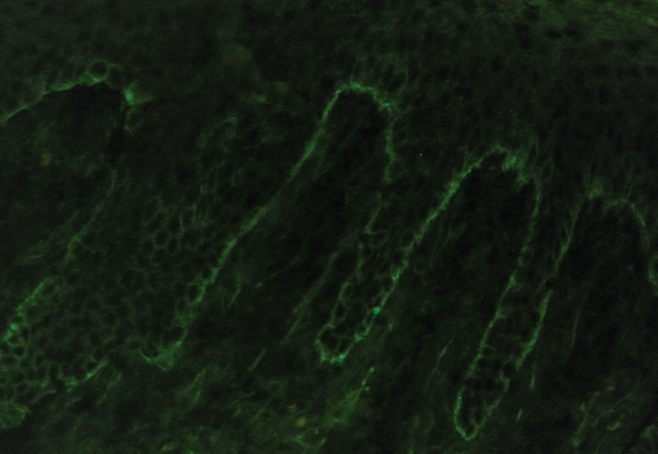
A biopsy was taken for evaluation with hematoxylin and eosin (H&E) staining and direct immunofluorescence (DIF). Pending the histopathology results, treatment was initiated with oral prednisone. After the patient had been on prednisone at 40 mg/day for 14 days his eruption was unchanged. The pathology report was received and showed eosinophils, subepidermal blistering, and lymphocytes on H&E. The DIF revealed dense granular and linear C3 and IgG deposition along the basement membrane (Figure 2) and faint deposits of C3 in the basal cell cytoplasm. The pathologist’s interpretation was “pemphigoid-like pattern, suggestive of drug-related etiology.
How would you approach further investigation in this case, considering that the patient reported being on a single medication for the past 5 years?
DISCUSSION
As reviewed in a published article presenting this case, the clinical appearance of the patient’s skin eruption raised suspicion of bullous pemphigoid (BP).1 Idiopathic BP typically develops in older adults (aged > 70 years).2 However, an independent association between ulcerative colitis and BP has been reported, and in one study, the association was seen especially in patients aged < 50 years.2-4
As noted by the pathologist’s report, a subepidermal blistering eruption can also develop as an adverse reaction to a prescription or OTC medication. Lesions of drug-related BP appear clinically similar or identical to those seen in idiopathic BP, and the 2 disorders also have overlapping histological features.5 Authors of a recent systematic review of drug-associated BP identified 89 drugs that had been implicated in drug-related BP. Mesalamine was included in the list, although its association with BP was categorized as “uncertain,” and its use by this patient was not initially suspected as the cause of his eruption, considering that he had been on the medication for several years.
Because patients may fail to mention OTC products, herbal supplements, or illicit drugs when queried about medication use, a more detailed history was pursued with the patient, asking specifically about oral or topical use of nonprescription agents and about any recent dietary changes that might suggest potential exposure to a new preservative. The patient denied using any other drugs or making changes in his diet. Upon further questioning about his use of mesalamine, the patient revealed that he had been ordering the medication online from a Canadian pharmacy as a cost-saving measure. Then he mentioned that for his most recent refill, he switched from using a brand-name product (Asacol), which is approved by both the FDA and Health Canada, to a less expensive generic mesalamine. The patient reported that he had begun taking the generic medication just a few weeks before he developed the blistering eruption.
The generic tablet, which was not approved by the US Food and Drug Administration and not available in the United States, releases 80% of the active ingredient at a pH of ≤ 6, thereby allowing systemic absorption of mesalamine through the stomach and small intestine.7
The patient was told to stop the generic 5-ASA. He restarted the brand-name 5-ASA product, and began a rapid taper to discontinue the prednisone. His bullous eruption resolved spontaneously within a few weeks and he had no recurrences during 8 months of available follow-up.
This case serves as a reminder about the potential risks associated with obtaining medications online from unverified sources or foreign pharmacies. Much of the concern relates to dealings with fake pharmacies distributing “medications” that are counterfeit, expired, or substandard in some other way. As illustrated by this case, however, ordering through legitimate pharmacies also poses potential risks.
Medications that are approved by regulatory agencies in other countries can differ in their formulation characteristics, with consequences for efficacy and/or safety. Even in the US, changing from a brand-name product to a generic version of the same medication or between generic versions can result in adverse reactions because generic products can be manufactured with different inactive ingredients. Therefore, a thorough medication history that probes for a possible product switch is always important when puzzled by a case involving a suspected drug-induced skin reaction.
Dr. Zirwas is Director, Ohio Contact Dermatitis Center Columbus, Ohio Member, North American Contact Dermatitis Group.
REFERENCES
- Ferris LK, Jukic D, English JC III, Zirwas MJ. Drug-induced bullous pemphigoid caused by a generic Canadian medication obtained over the internet. Arch Dermatol. 2005;141(11):1474-1476.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepidermal autoimmune blistering dermatoses: Clinical features and diagnosis. J Am Acad Dermatol. 2021; Mar 5:S0190-9622(21)00475-8. doi:10.1016/j.jaad.2020.11.076. Epub ahead of print.
- Chen YJ, Juan CK, Chang YT, et al. Association between inflammatory bowel disease and bullous pemphigoid: a population-based case-control study. Sci Rep. 2020;10(1):12727.
- Narla S, Silverberg JI. Associations of pemphigus or pemphigoid with autoimmune disorders in US adult inpatients. J Am Acad Dermatol. 2020;82(3):586-595.
- Verheyden MJ, Bilgic A, Murrell DF. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
- Asacol. Package insert. Allergan; 2020.
- Sauriol C, Comtois F. Relative in vitro dissolution profiles of Salofalk, Asacol, and Pentasa 5-ASA tablets: proceedings of the 5th Falk Symposium. In: Williams CN, ed. Trends in Inflammatory Bowel Disease Therapy. Dordrecht. The Netherlands: Kluwer Academic Publishers; 1991;441-442.
DISCLOSURES
Dr. Zirwas reports no relevant financial interests.