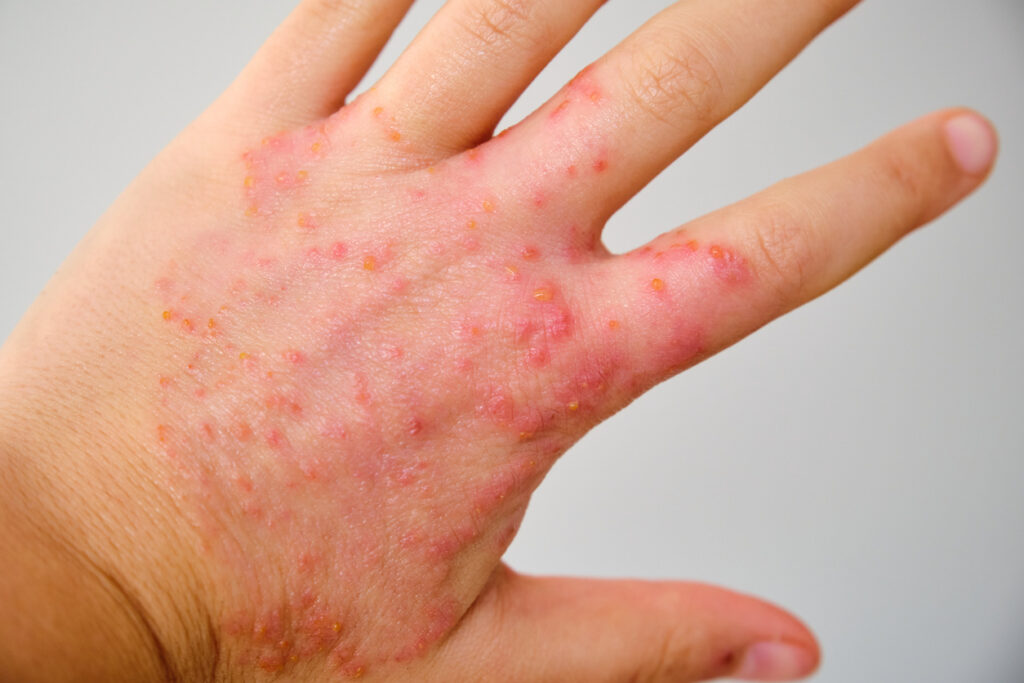
Health Canada has approved delgocitinib cream 20mg/g (Anzupgo, LEO Pharma) for the treatment of adults (aged ≥18 years) with moderate-to-severe chronic hand eczema (CHE) for whom topical corticosteroids are inadequate or are not advisable.
Delgocitinib cream 20mg/g is currently expected to be available to patients and healthcare professionals in Canada in 2025.
It is the first topical treatment specifically indicated for CHE, which affects approximately 4.7% of the population globally, and an estimated 6% of Canadians, LEO Pharma notes. Delgocitinib cream is also the first topical pan-Janus kinase (JAK) inhibitor to be approved in Canada specifically for moderate-to-severe CHE. It inhibits the activation of the JAK-STAT signaling pathway, which plays a crucial role in the pathogenesis of CHE.
Based on DELTA 1 and 2 Results
The Health Canada approval is based on the results from the DELTA 1 and 2 clinical trials, which included 960 adult patients with moderate-to-severe CHE (20% of whom were Canadian). Both trials met their primary and key secondary endpoints and showed that delgocitinib cream provided overall superior efficacy vs. cream vehicle and was well-tolerated over 16 weeks.
“Living with chronic hand eczema can significantly impact a patient’s quality of life, affecting their ability to perform daily activities and their overall well-being,” says Melinda Gooderham, MSc, MD, FRCPC, Dermatologist and Medical Director at SKiN Centre for Dermatology and Principal Investigator for the SKiN Research Centre in Peterborough, Ontario, Canada, in a news release. “The approval of Anzupgo provides a new treatment option that targets underlying inflammation and helps manage the symptoms of this debilitating condition.”
“Hand eczema can have a profound effect on everyday living and can impact an individual’s everyday tasks, family life, ability to work, and mental health,” adds Amanda Cresswell-Melville, Executive Director of the Eczema Society of Canada. “More treatment options means more hope for those impacted by this challenging condition.”
Delgocitinib cream is currently approved in the United States, European Union, United Kingdom, Switzerland, and the United Arab Emirates under the brand name Anzupgo for the treatment of moderate-to-severe CHE in adults for whom topical corticosteroids are inadequate or inappropriate.