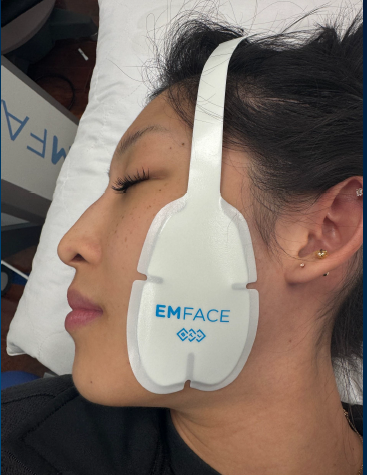
The U.S. Food and Drug Administration has cleared BTL’s Emface platform for the treatment of temporomandibular joint (TMJ) dysfunction.
Emface is now cleared by the FDA for the relief of symptoms associated with muscle spasm, treatment of TMJ dysfunction and associated pain, muscle re-education, increased local blood flow, and the maintenance or improvement of mandibular range of motion.
Emface employs synchronized radiofrequency technology to gently heat the dermis. Concurrently, the high-intensity focused electrical stimulation (HIFES) selectively contracts facial muscles.
“EMFACE is an established technology platform with multiple FDA-cleared uses,” says David Chmel, CEO of BTL Industries, in a news release. “This new indication allows dental, oral health, and wellness providers to address TMJ dysfunction within its approved uses, expanding the benefits of EMFACE to more patients.”
“TMJ dysfunction impacts more than the jaw—it affects quality of life,” adds Shireen Dhanani, DMD, Leesburg, FL. “With Emface, we can now offer an accessible, non-invasive option that not only helps patients find relief and functional improvement, but also expands the scope of dental care.”