Alexander Egeberg, MD, PhD, with Bob Kronemyer
Topical corticosteroids (TCSs) are some of the most frequently used drugs for skin diseases worldwide. While these agents are generally considered safe, they are often prescribed with little thought about the dosage or duration.
Systemic and inhaled corticosteroids negatively impact bone remodeling and cause osteoporosis and bone fracture when prescribed continuously or in high doses. However, risk of osteoporosis and a major osteoporotic fracture after application of TCSs is largely unexplored.
From clinical experience, my colleagues and I have observed patients who develop a cushingoid appearance or individuals with diabetes who end up with dysregulated blood sugar following use of potent TCSs on large body surface areas.
Upon scrutinizing the published literature, we observed a noticeable data gap about the safety of these therapies. Also, when we contacted the manufacturers of the drugs, we were surprised to learn that most of them had no data on the systemic effects of these medications.
Because these drugs are used by millions of people, even a small risk is important from a public health perspective. We therefore decided to undertake a study to assess the potential for systemic effects of TCSs.
The Danish study
The nationwide retrospective Danish study gleaned data from the Danish National Patient Registry, which contains patient information from all inpatient and outpatient visits at all Danish hospitals and a number of private clinics.1
The study analyzed a total of 723,251 adults, 52.8% women and 47.2% men, all aged 18 and older, who were alive and residents of Denmark from 2003 to 2017. During the study period, they all filled prescriptions of at least 200 g of mometasone furoate (1 mg/g) or the equivalent amount in equipotent doses of other potent or very potent TCSs.
During this span of 15 years, 25.8% of patients were categorized as exposed to 500 g to 999 g of mometasone or equivalent; 15.4% to 1000 g to 1999 g; 13.0% to 2000 g to 9999 g; and 1.9% to at least 10,000 g.
For example, each time the amount of TCSs doubles—for example, from 100 g to 200 g or from 400 g to 800 g—the relative risk of osteoporosis increases by 3%.
The study concluded that the hazard ratios (HRs) of a major osteoporotic fracture were 1.01 for exposure to 500 g to 999 g of mometasone or equivalent; 1.05 for exposure to 1000 g to 1999 g; 1.10 for exposure to 2000 g to 9999 g; and 1.27 for exposure to at least 10,000 g (see Figure).
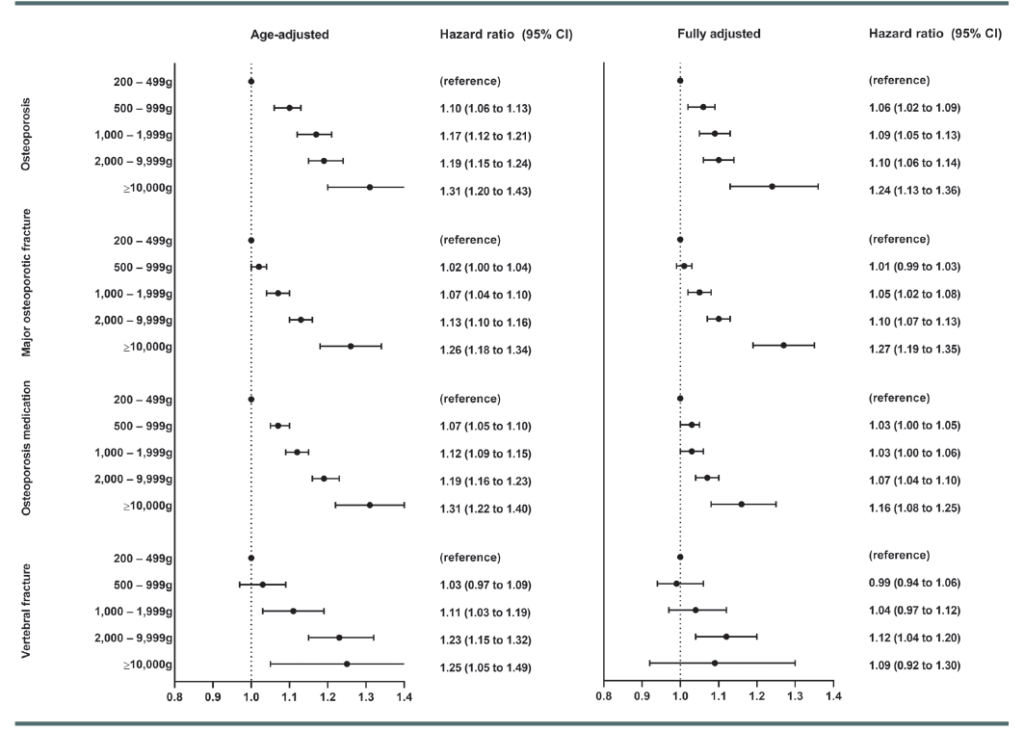
Moreover, our results showed that 4.3% of all cases of osteoporosis are due to TCSs. However, it is very important to emphasize that the absolute risk to the individual patient (in other words, the average topical corticosteroid user) is still very low. For a major osteoporotic fracture, 2.7% of all fractures were attributable to TCS use.
The lowest exposure needed for one additional patient to develop a major osteoporotic fracture in 454 person-years was an exposure of at least 10,000 g of mometasone or equivalent, which is 2 to 3 times higher than what is seen with oral corticosteroids.
The specific incidence rates for a major osteoporotic fracture were 81.6 new cases per 10,000 person-years for 200 g to 499 g of mometasone; 88.7 cases per 10,000 person-years for 500 g to 999 g; 100.6 cases per 10,000 person-years for 1000 g to 1999 g; 113.1 cases per 10,000 person-years for 2000 g to 9999 g; and 122.2 cases per 10,000 person-years for at least 10,000 g.
The incidence rate of osteoporosis among patients treated with the equivalent of 200 g to 499 g of mometasone was 36.7 new cases per 10,000 person-years; 43.1 cases per 10,000 person-years for 500 g to 999 g; 50.2 cases per 10,000 person-years for 1000 g to 1999 g; 55.2 cases per 10,000 person-years for 2000 g to 9999 g; and 58.7 cases per 10,000 person-years for at least 10,000 g.
Our results confirm what we have probably long suspected, but never had any data on: that there may be clinically relevant systemic effects of TCSs in large quantities.
The outcomes also remained consistent across a wide range of sensitivity analyses and after adjusting for potential confounding factors. Women younger than 50, though, had slightly higher risk estimates linked to TCS than did older women.
Study takeaways
The most important takeaway from the study is that the risk to the average user of TCSs is extremely low. However, our study demonstrates that there are indeed systemic effects when using these drugs. We used a very hard-to-achieve endpoint in our study, but other effects, such as the potential for dysregulated diabetes, is something that we may need to be aware of when prescribing these therapies.
In some cases, the risk of bone disease for a specific total cumulative topical steroid dose may be greater than the benefit of continuing topical therapy.
As dermatologists, we do not prescribe these drugs for fun, but rather we prescribe them to patients who need an effective therapy. As such, there is no specific threshold for “too much TCS use,” and TCSs remain a valuable tool for all dermatologists. However, in patients who have used vast amounts of potent TCSs on large body surface areas or for prolonged periods, such as for skin diseases including atopic dermatitis and psoriasis, it may be prudent to consider whether there is a suitable treatment alternative, such as a systemic therapy or a steroid-sparing topical drug.
In some cases, alternative pharmaceutical therapies exist. If not, it might be worth recommending oral prophylaxis with calcium and vitamin D for these patients, and considering a DXA scan in some instances.
One limitation of the study is that we lacked weight and smoking status for patients, yet we found comparable estimates among patients with and without psoriasis, which is a condition strongly linked to obesity and smoking.
Surveillance bias is also a concern with observational register-based studies, but when analyzing our data we did not find evidence of surveillance bias. Furthermore, our endpoint of major osteoporotic fracture is unlikely to be affected by surveillance because it is considered a very “hard endpoint.”
TCSs are rightfully here to stay; however, as more and more novel treatment alternatives become available, it will be interesting to see data on whether use of these novel treatment options can lead to a more modest use of TCSs, such as in patients with severe atopic dermatitis.
Dr. Egeberg is a Dermato-epidemiologist in the Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark.
References
1. Egeberg A, Schwarz P, Harslof T, e al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157(3):275-282. doi:10.1001/jamadermatol.2020.4968
Disclosures
Dr. Egeberg reports no relevant financial interests.