Galderma is launching two new clinical trials to investigate the efficacy and safety of nemolizumab (Nemluvio) in treating patients living with Systemic Sclerosis (SSc) and Chronic Pruritus of Unknown Origin (CPUO).
Galderma’s Phase 2 proof-of-concept study is a multicenter, randomized, double-blind, placebo-controlled study investigating the pharmacokinetics and pharmacodynamics of the interleukin(IL)-31 blocker in adults with SSc. Patient enrollment is planned to begin in H2 2025, with completion anticipated in 2028.
Designed with Steering Committee
The study was designed in collaboration with a Steering Committee of rheumatologists and dermatologist, including lead trial investigator, Professor Oliver Distler, MD, Zürich, Switzerland; Professor Dinesh Khanna, MD, Director of the Scleroderma Program, University of Michigan, United States (U.S.); Professor Robert Spiera, MD, Director of the Scleroderma, Vasculitis and Myositis Center, Hospital for Special Surgery, New York, U.S.; and Professor Johann Gudjonsson, MD, PhD, Dermatologist, University Hospital Michigan, U.S.
The trial is expected to be conducted in several countries in North America, Europe, and South America. More information about the study will be made available soon on the clinicaltrials.gov website.
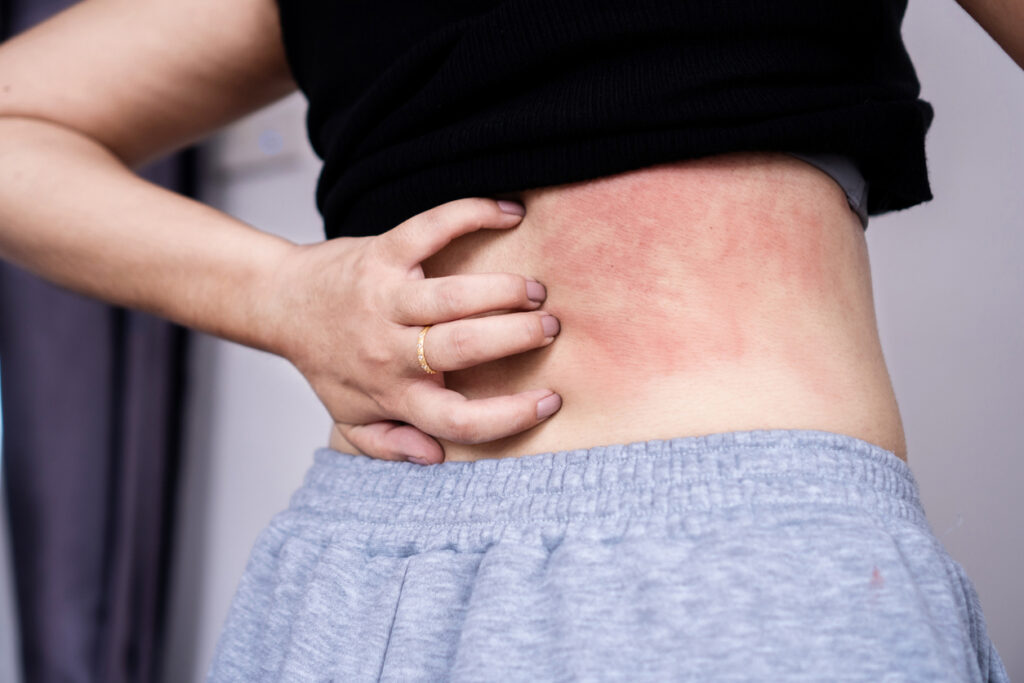
“Systemic Sclerosis can have a profound impact on both the quality and length of a person’s life. It causes the skin to harden, damages blood vessels, leads to joint pain, and can result in serious fibrosis in multiple internal organs, sometimes with life-threatening consequences,” says Dr. Distler, Professor of Rheumatology at the University of Zurich and Head of the Department of Rheumatology at the University Hospital Zurich, in a news release. “With no currently approved treatments that are indicated to treat the several symptoms this autoimmune disease presents, I look forward to investigating the role that nemolizumab could potentially play in this condition.”
Phase 2 CPUO Trial
Galderma’s new Phase 2 CPUO trial will explore the pharmacokinetics and pharmacodynamics of nemolizumab in adults. Enrollment is expected to start in H2 2025 in the U.S., with completion anticipated in 2026. The study was designed in collaboration with a Steering Committee of dermatologists including the lead investigator Dr. Shawn Kwatra, MD, PhD, Joseph W. Burnett Endowed Professor, Chairman of Dermatology, University of Maryland School of Medicine, U.S., and Dr. Sarina Elmariah, MD, PhD, MPH, Associate Professor and Dermatology Director at the Center for Itch and Neurosensory Disorders at the University of California in San Francisco, U.S.
The study is being conducted in the U.S., and more information about the study will be made available soon on the clinicaltrials.gov website.
“It is challenging to treat Chronic Pruritus of Unknown Origin as physicians have limited therapeutic options specifically targeting the underlying cause of itch,” says Dr. Kwatra. “With the extensive data showing that IL-31 is a key driver of itch, I’m excited to explore whether nemolizumab’s inhibition of IL-31 signaling might effectively reduce the intractable itch experienced by patients with Chronic Pruritus of Unknown Origin.”