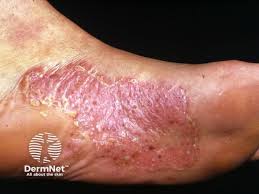
LEO Pharma is initiating a Phase 2a proof-of-concept trial to evaluate the efficacy and safety of delgocitinib cream in the treatment of adults with mild-to-severe Palmoplantar Pustulosis (PPP).
Delgocitinib cream is a topical pan-Janus kinase (JAK) inhibitor shown to inhibit the activity of all four Janus kinases (JAKs), which are involved in inflammatory signaling. Using delgocitinib cream’s mechanism of action, the trial will investigate the potential benefit of inhibiting multiple JAKs to treat PPP.
DELTA NEXT Trial
DELTA NEXT is a Phase 2a, randomized, double-blind, two-arm, vehicle control, adaptive design proof-of-concept trial to assess the efficacy and safety of twice-daily delgocitinib cream compared with cream vehicle in adult subjects with mild to severe PPP. The DELTA NEXT trial plans to recruit up to 135 patients with active, chronic, mild to severe PPP for whom topical corticosteroids are inadequate or inadvisable. Participants will be recruited across 40-45 sites in the United States, Canada, the United Kingdom, Germany, and Poland.
“The DELTA NEXT trial in PPP represents an exciting new chapter in the clinical development journey of delgocitinib cream,” says Christophe Bourdon, Chief Executive Officer, LEO Pharma, in a news release. “PPP is a disease with few treatment options that can severely affect quality of life. We are hopeful that this therapy could potentially provide much-needed help for patients suffering from this devastating condition.”
“I’ve seen firsthand how PPP can severely disrupt patients’ lives, making it painful to walk, perform activities of daily living and impacting their ability to work,” adds Dr. Robert Bissonnette, international coordinating investigator (ICI) on the DELTA NEXT trial and Chairman at Innovaderm Montreal, Canada. “Despite the significant burden it places on patients, treatment options remain limited. There is a real and urgent need for effective therapies.”
Currently Approved for CHE
Currently, delgocitinib cream is approved for adults in the European Union, United Kingdom, Switzerland, and the United Arab Emirates for the treatment of moderate-to-severe CHE for whom topical corticosteroids are inadequate or inappropriate, and is under review in other markets, including the United States.
PHOTO CREDIT: DermNet