The U.S. Food and Drug Administration (FDA) has approved the supplemental New Drug Application (sNDA) for roflumilast topical foam 0.3% (Zoryve, Arcutis) for the treatment of plaque psoriasis of the scalp and body in adult and pediatric patients 12 years of age and older.
The approval is supported by positive results from Arcutis’ Phase 2 and pivotal Phase 3 trials in plaque psoriasis. The “A Randomized tRial Employing topiCal roflumilasT foam to treat scalp psORiasis” (ARRECTOR) and the Phase 2 (Trial 204) were multicenter, randomized, double-blind, vehicle-controlled studies evaluating the safety and efficacy of roflumilast topical foam 0.3% in plaque psoriasis. Together, the two studies enrolled 736 adults and adolescents ages 12 years and older with mild-to-severe plaque psoriasis of scalp and body. In each trial, subjects were randomized 2:1 to receive roflumilast topical foam 0.3% or vehicle foam applied once daily for 8 weeks.
Met Endpoints
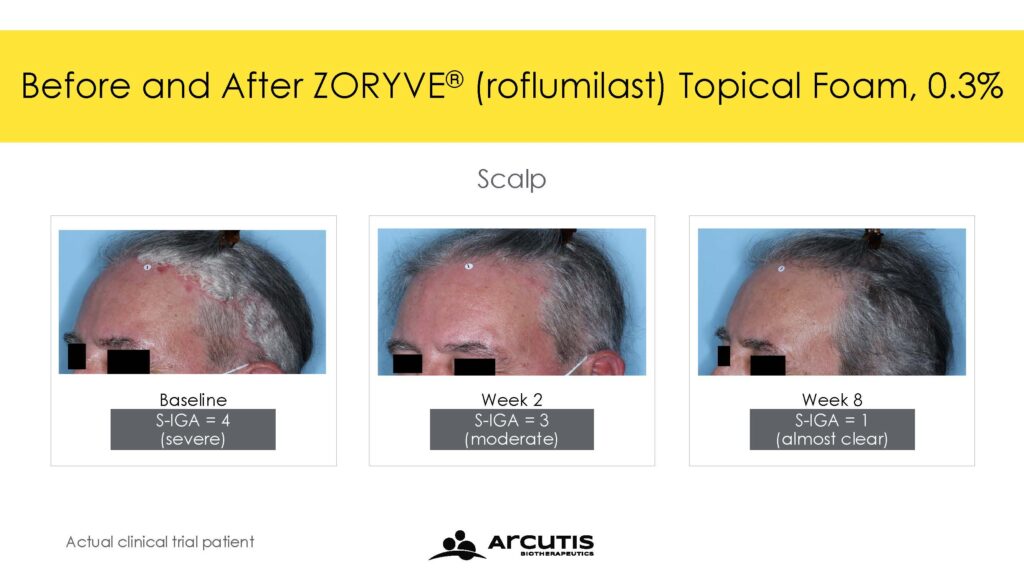
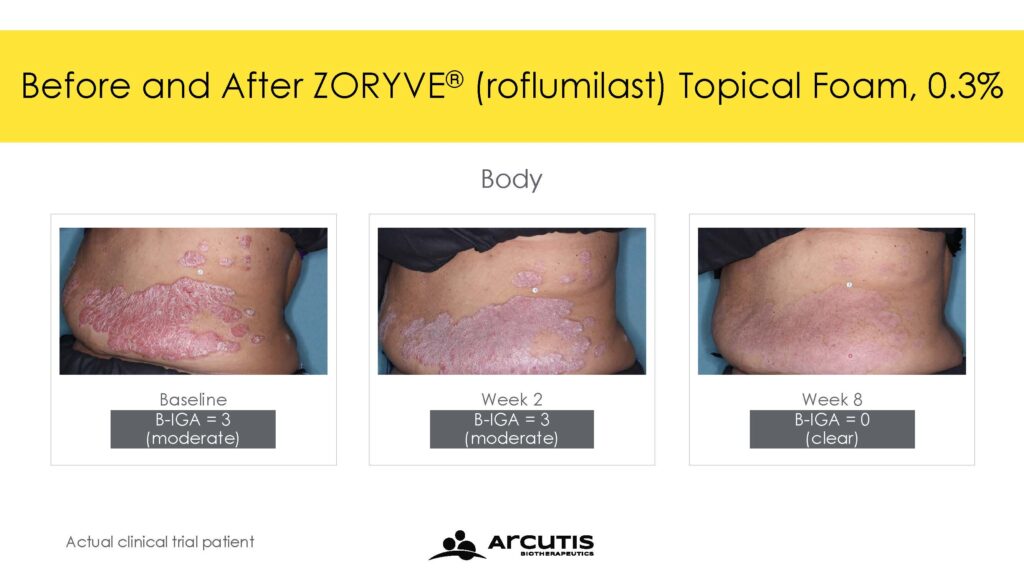
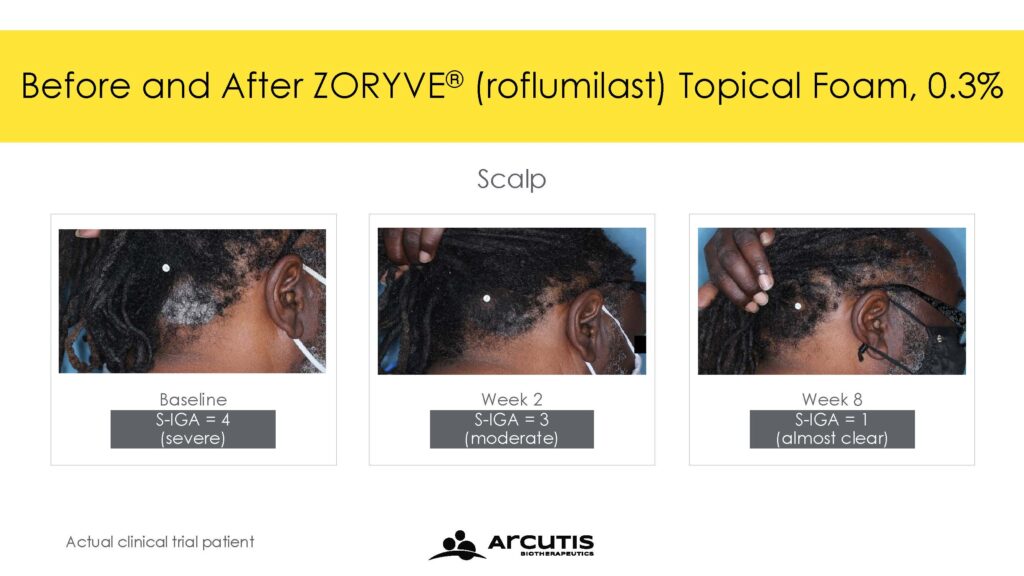
The ARRECTOR study met its co-primary endpoints of Scalp-Investigator Global Assessment (S-IGA) Success and Body-Investigator Global Assessment (B-IGA) Success. For Scalp-IGA, 66.4% of individuals treated with roflumilast topical foam 0.3% compared to 27.8% of individuals treated with a matching vehicle foam achieved Scalp-IGA Success at Week 8. For Body-IGA, 45.5% of individuals treated with roflumilast topical foam 0.3% compared to 20.1% of individuals treated with a matching vehicle foam achieved Body-IGA Success at Week 8. IGA Success was defined as an IGA score of ‘clear’ or ‘almost clear’ plus a 2-point improvement from baseline.
Trial 204 met its primary endpoint with 56.7% of individuals treated with roflumilast topical foam 0.3% achieving S-IGA Success compared to 11.0% of individuals treated with a matching vehicle foam at Week 8. In addition, 39.0% of individuals treated with roflumilast topical foam 0.3% achieved B-IGA Success compared to 7.4% of individuals treated with a matching vehicle foam at Week 8.
Itch Improvement
Roflumilast topical foam 0.3% foam provided a clinically meaningful improvement in itch. In ARRECTOR, two thirds (65.3%) of individuals treated with roflumilast achieved a clinically significant reduction in itch compared to approximately one third (30.3%) of individuals treated with vehicle at Week 8 as measured by a ≥ 4-point change from baseline in Scalp Itch-Numeric Rating Scale (SI-NRS). Importantly, a greater improvement in scalp itch was observed 24 hours following the first application with roflumilast foam compared to vehicle. The improvement in scalp itch was consistent in Trial 204, with a higher percentage of individuals achieving SI-NRS Success at Week 8 with roflumilast foam compared to vehicle (67.3% vs. 20.7%).
In addition, the roflumilast foam also provided improvement in body itch as measured by the Worst Itch-Numeric Rating Scale (WI-NRS), with 63.1% of those treated with roflumilast foam achieving a ≥ 4-point reduction in WI-NRS compared to 30.1% of those treated with vehicle at Week 8 in ARRECTOR.
Roflumilast topical foam 0.3% was well tolerated. The incidence of Treatment Emergent Adverse Events (TEAEs) was low and generally similar between active treatment and vehicle, with most TEAEs assessed as mild to moderate severity. Overall, the most common adverse reactions for roflumilast foam for plaque psoriasis in the Phase 3 and Phase 2 studies combined (≥1%) included headache (3.1%), diarrhea (2.5%), nausea (1.7%), and nasopharyngitis (1.3%). Rates of discontinuation due to adverse events were low and similar among roflumilast-treated and vehicle-treated patients in pooled vehicle-controlled studies.
“Living with plaque psoriasis can have a profound impact on people’s emotional well-being, quality of life, and social relationships. This can be even further exacerbated when psoriasis appears on the face, scalp, or thin-skinned areas,” says Leah Howard, President and Chief Executive Officer of the National Psoriasis Foundation. “We are pleased to see new advancements and innovation in treatments for the millions afflicted with this serious skin disease, that can be used long-term and anywhere the disease presents.”
Improving Access to Roflumilast
Roflumilast topical foam 0 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via wholesaler and dermatology pharmacy channels. The ZORYVE Direct Program helps patients access their prescribed Arcutis medication. Specifically, this patient support program helps those who have been prescribed roflumilast to navigate the payer process, assists patients with adherence, and includes the roflumilast Direct Savings Card Program, which can help reduce out-of-pocket costs for eligible commercially insured patients. Arcutis will also continue to offer the Arcutis Cares™patient assistance program (PAP) that provides roflumilast at no cost for financially eligible patients who are uninsured or underinsured.

