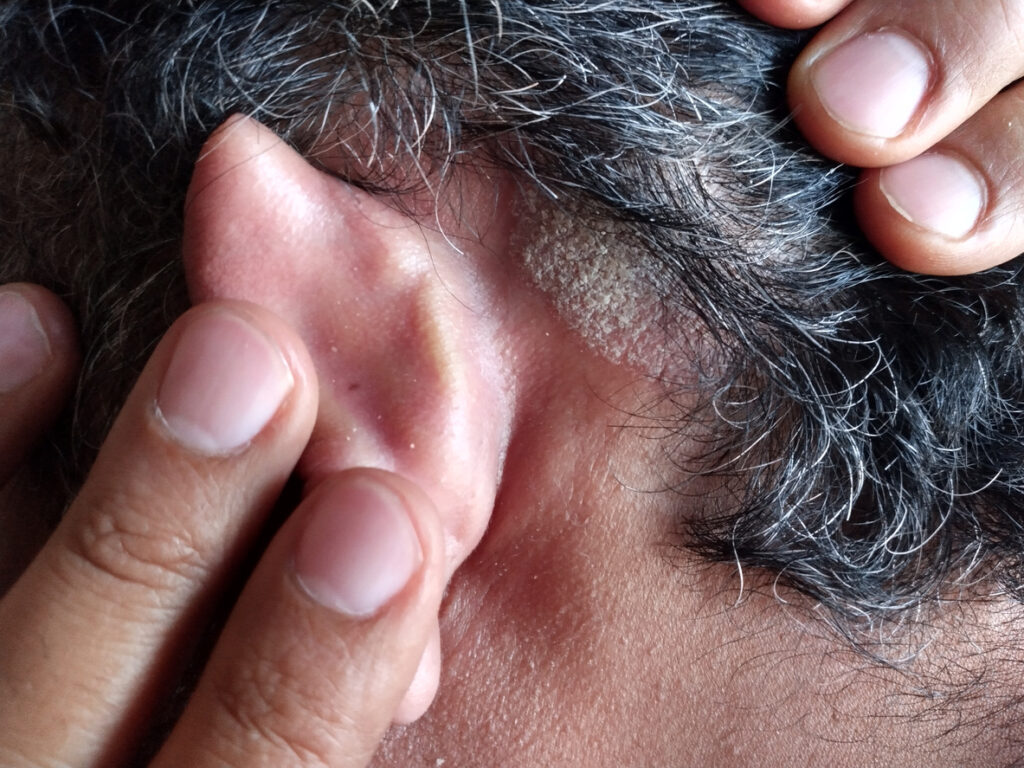
Icotrokinra, Johnson & Johnson’s investigational oral interleukin(IL)-23 blocker, shows significant skin clearance in patients with difficult-to-treat scalp and genital psoriasis, according to new Phase 3 data presented at the 2025 Society for Investigative Dermatology (SID) Annual Meeting in San Diego, CA.
The ICONIC-TOTAL is a Phase 3 randomized controlled trial evaluating the efficacy and safety of icotrokinra compared with placebo for the treatment of plaque PsO in 311 participants (icotrokinra=208; placebo=103) with at least moderate severity affecting special areas (e.g., scalp, genital, and/or hands and feet) with overall Investigator’s Global Assessment (IGA) score of 0 or 1 with at least a 2-grade improvement as the primary endpoint. It included adults and adolescents aged 12 and older with body surface area as low as 1% and at least moderate plaque psoriasis (PsO) affecting high-impact skin sites.
Fully 57% of patients treated with once daily icotrokinra achieved the study’s primary endpoint with an Investigator’s Global Assessment (IGA) score of 0/1 (clear or almost clear skin) and a ≥2-grade improvement from baseline at Week 16 compared to 6% of patients receiving placebo.
High Rates of Skin Clearance
Icotrokinra demonstrated high rates of skin clearance in patients with scalp psoriasis as 66% achieved a scalp-specific Investigator’s Global Assessment (ss-IGA) score of 0/1 compared to 11% receiving placebo at Week 16. At the same time point, among patients with genital psoriasis, 77% treated with icotrokinra achieved a static Physician’s Global Assessment of Genitalia (sPGA-G) score of 0/1 compared to 21% receiving placebo. In the smaller subset of patients with hand/foot psoriasis, treatment with icotrokinra showed a numerically higher rate of skin clearance at Week 16 with 42% achieving a hand and/or foot Physician’s Global Assessment (hf-PGA) score of 0/1 compared to 26% receiving placebo.
“While plaque psoriasis can appear in any location on the body, most high-impact skin sites affect areas critical for mobility, personal care, and intimacy, and can be very challenging to treat effectively. Notably, almost 80% of psoriasis patients experience scalp involvement,” says Melinda Gooderham, MSc, MD, FRCPC, SKiN Centre for Dermatology, Queen’s University, and Probity Medical Research, Peterborough, ON, Canada and ICONIC-TOTAL study investigator, in a news release. “Results from the ICONIC-TOTAL study demonstrate impressive rates of skin clearance in these difficult-to-treat areas and show the potential for treatment with icotrokinra to offer patients a novel therapeutic option that aligns with their treatment needs and preferences.”
Favorable Safety Profile
Icotrokinra demonstrated a favorable safety profile. A similar proportion of patients experienced adverse events (50% and 42%) and serious adverse events (0.5% and 1.9%) in icotrokinra and placebo respectively through Week 16, with no new safety signals identified.
“When plaque psoriasis affects sensitive areas of the body, patients often experience unique challenges that can have a profound impact on their daily lives,” says Liza O’Dowd, MD, Vice President and Immunodermatology Disease Area Lead at Johnson & Johnson Innovative Medicine. “These new findings build upon the impressive scalp psoriasis results seen in ICONIC-LEAD and strengthen the breadth of data demonstrating the potential for icotrokinra to shift the treatment paradigm in moderate-to-severe plaque psoriasis, by offering a combination of skin clearance and favorable safety in a once daily pill.”