The Centers for Medicare and Medicaid Services (CMS) issued a permanent, product-specific billing code for Fresenius Kabi’s Otulfi (ustekinumab-aauz), an ustekinumab biosimilar referencing Stelara.
Under the Healthcare Common Procedure Coding System (HCPCS), the Q-code Q9999 is for “Injection, for subcutaneous use or intravenous use, ustekinumab-aauz (Otulfi), biosimilar per 1.0mg.”
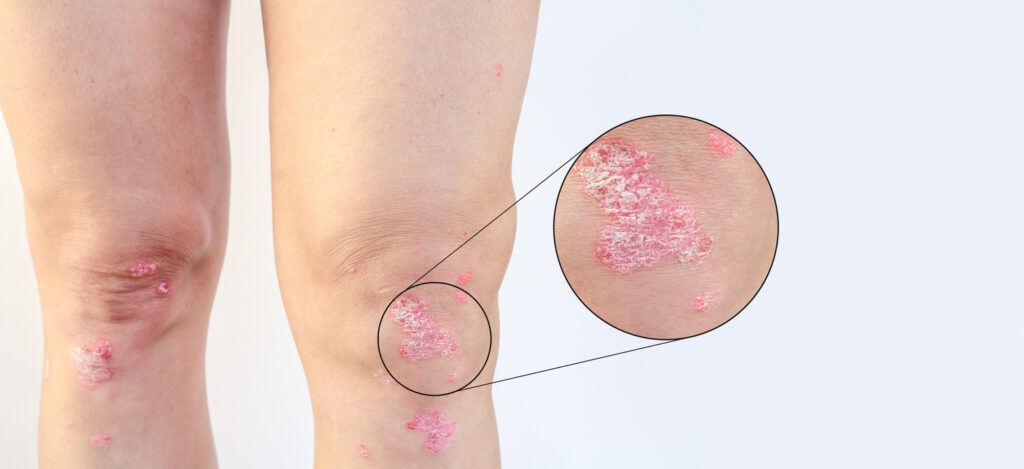
Otulfi (ustekinumab-aauz) is U.S. Food and Drug (FDA)-approved for subcutaneous and intravenous formulations to treat the same conditions as the reference product Stelara (ustekinumab), including moderate-to-severe plaque psoriasis, active psoriatic arthritis, Crohn’s disease, and ulcerative colitis. A Q-code is a CMS reimbursement code used by healthcare providers and payers to process claims for drugs administered through injection, infusion, or other means.
Commercial insurers and government payers use them to standardize claims submissions and reimbursements. Pass-through payments are used by hospital outpatient department services through the Hospital Outpatient Prospective Payment System (OPPS) to support use of new devices, drugs, and biologicals that meet eligibility criteria for a period of at least two years but not more than three years.