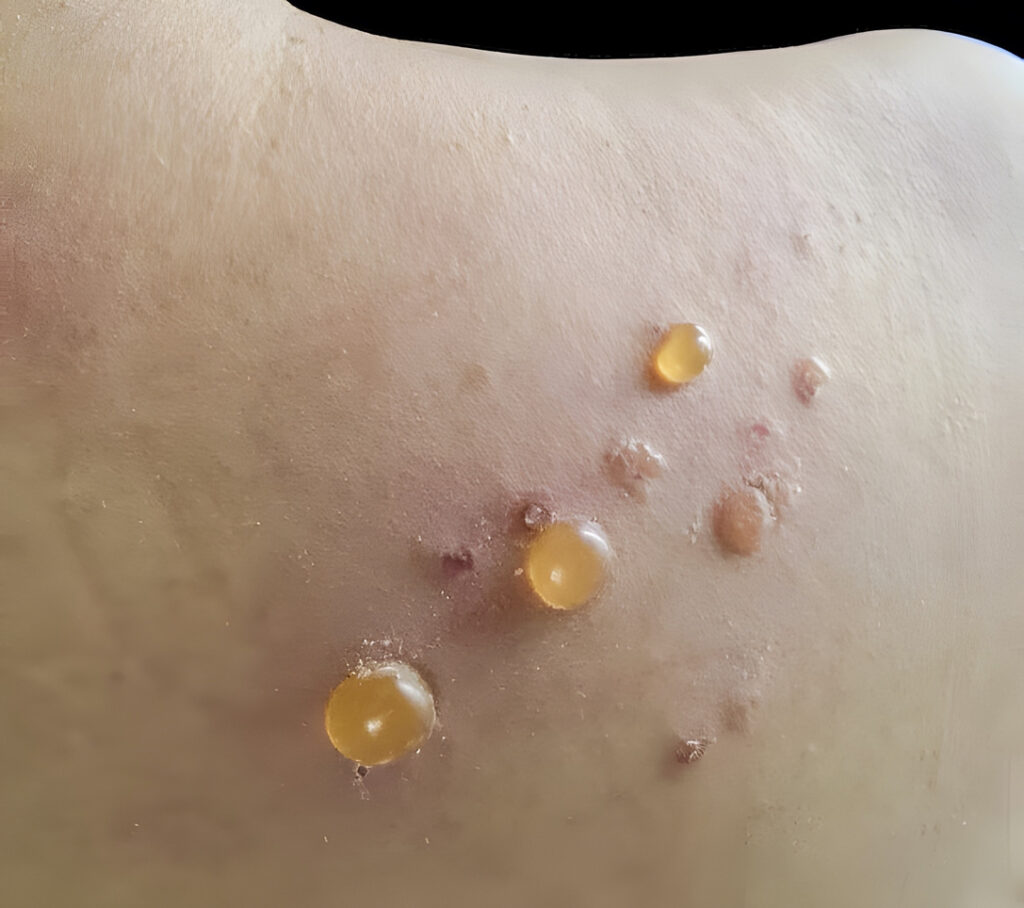
Dupilumab (Dupixent, Sanofi & Regeneron) hit all the key primary and secondary endpoints in a study of adults with Bullous Pemphigoid (BP).
Results from the pivotal ADEPT Phase 2/3 trial were shared in a late-breaking oral presentation at the 2025 American Academy of Dermatology (AAD) Annual Meeting in Orlando, FL.
In February 2025, the U.S. Food and Drug Administration (FDA) accepted for Priority Review the supplemental Biologics License Application for dupilumab to treat BP. The FDA decision is expected by June 20, 2025.
Dupilumab was previously granted Orphan Drug Designation by the FDA for BP. Additional applications are also under review around the world, including in the European Union. The safety and efficacy of dupilumab in BP are currently under clinical investigation and have not been evaluated by any regulatory authority.
The ADEPT trial met all primary and key secondary endpoints, enrolling 106 adults with moderate-to-severe BP who were randomized to receive dupilumab 300mg (n=53) every two weeks after an initial loading dose or placebo (n=53) added to standard-of-care oral corticosteroids (OCS). During treatment, all patients underwent a protocol-defined OCS tapering regimen if control of disease activity was maintained. Sustained disease remission was defined as complete clinical remission with completion of OCS taper by week 16 without relapse and no rescue therapy use during the 36-week treatment period.
Results for dupilumab-treated patients at 36 weeks, compared to those treated with placebo, were as follows:
- 20% experienced sustained disease remission, the primary endpoint, compared to 4%.
- 40% achieved ≥90% reduction in disease severity compared to 10%.
- 40% achieved clinically meaningful itch reduction compared to 11%.
- 1678mg reduction in cumulative OCS exposure on average and a 54% lower risk of rescue medication use.
In this elderly population, overall rates of adverse events (AEs) were 96% (n=51) for dupilumab and 96% (n=51) for placebo. AEs more commonly observed with dupilumab compared to placebo in at least 3 patients included peripheral edema (n=8 vs. n=5), arthralgia (n=5 vs. n=3), back pain (n=4 vs. n=2), blurred vision (n=4 vs. n=0), hypertension (n=4 vs. n=3), asthma (n=4 vs. n=1), conjunctivitis (n=4 vs. n=0), constipation (n=4 vs. n=1), upper respiratory tract infection (n=3 vs. n=1), limb injury (n=3 vs. n=2) and insomnia (n=3 vs. n=2). There were no AEs leading to death in the dupilumab group and two AEs leading to death in the placebo group.
“People with bullous pemphigoid live with unrelenting itch, blisters, and painful lesions that can be debilitating and make it difficult to function daily. Moreover, current treatment options can be challenging for this primarily elderly patient population because they work by suppressing their immune system,” says study author Victoria Werth, MD, Chief of the Division of Dermatology at the Philadelphia Veterans Administration Hospital, Professor of Dermatology and Medicine at the Hospital of the University of Pennsylvania and the Veteran’s Administration Medical Center, in Philadelphia, PA, in a news release. “By targeting the underlying type 2 inflammation, which is a key driver for bullous pemphigoid, Dupixent is the first investigational biologic to show sustained disease remission and reduce disease severity and itch compared to placebo in a clinical trial.”