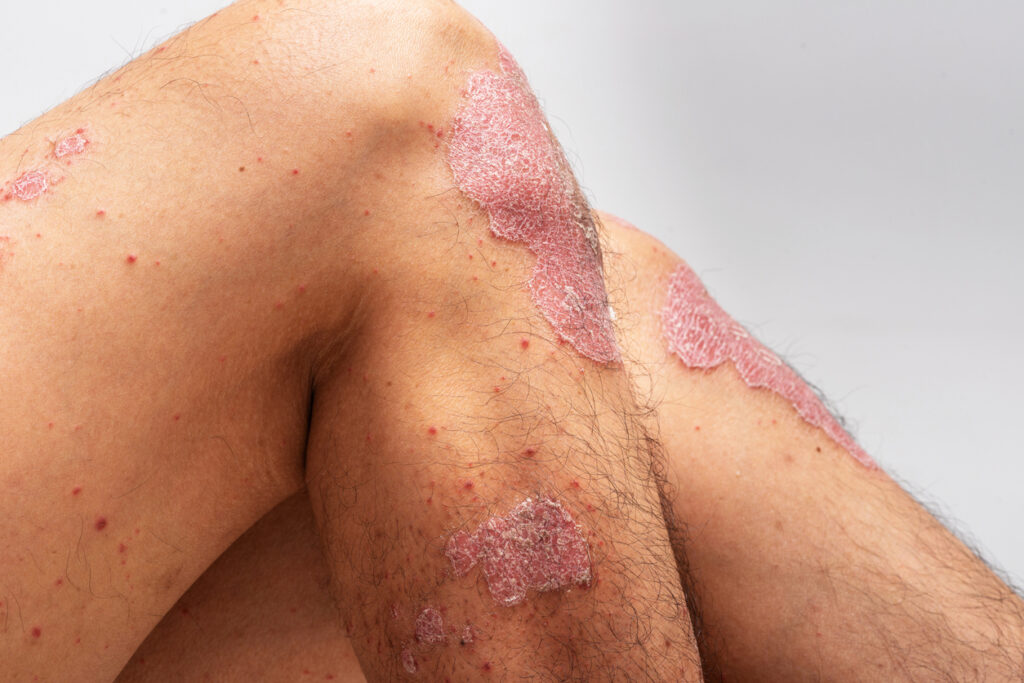
Deucravacitinib (Sotyktu, Bristol Myers Squibb) showed consistent safety and efficacy in patients with in moderate-to-severe plaque psoriasis for up to five years, new research shows.
In patients who were treated continuously with deucravacitinib, clinical response rates were maintained from Year 1 to Year 5, including Psoriasis Area and Severity Index (PASI) 75, PASI 90 and static Physician’s Global Assessment (sPGA) 0/1 (clear/almost clear). Moreover, the safety profile of deucravacitinib remained consistent through five years with no new safety signals identified.
Clinical efficacy outcomes were sustained in patients who were continuously treated with deucravacitinib for PASI 75 (72.1%, Year 1; 67.3%, Year 5), PASI 90 (45.9%, Year 1; 46.3%, Year 5) and sPGA 0/1 (57.5%, Year 1; 52.6%, Year 5).
ICYMI: The Dermatology Digest recently reported on a new expert consensus recommendations on the use of deucravacitinib in psoriasis.
“Today’s findings demonstrate the continued long-term safety and efficacy profile of Sotyktu, with patients maintaining skin clearance over five years,” says Mark Lebwohl, MD, dean of Clinical Therapeutics at the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai and an investigator and paid consultant for Bristol Myers Squibb, in a news release. “These results further support the role of Sotyktu, the first TYK2 inhibitor available for patients living with moderate-to-severe plaque psoriasis, as a potential oral standard of care.”
The efficacy analysis included 513 patients who received continuous deucravacitinib treatment from Day 1 in the pivotal PrOgram to Evaluate the efficacy and safety of Sotyktu (deucravacitinib), a selective TYK2 inhibitor (POETYK) PSO-1 and POETYK PSO-2 trials and transitioned to the POETYK PSO-LTE trial, while the safety analysis included 1,519 patients who received at least one dose of deucravacitinib during the trials. The patients in this five-year analysis completed 256 weeks of treatment. Efficacy was analyzed using the modified nonresponder imputation (mNRI) method.
These data were presented at the Winter Clinical Dermatology Conference – Hawaii (WCH) in Big Island, Waikoloa Village, HI.