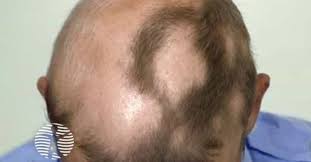
Thermo Fisher Scientific Inc is launching the international CorEvitas Adolescent Alopecia Areata (AA) Registry.
Data collected will enable research to help better understand the burden of disease for AA patients, as well as the real-world effectiveness and safety of newly approved treatments.
The registry is now active in both Europe and the U.S., with the first patient recently enrolled in Europe and the first patient in North America enrolled in late 2024. This is the 12th independent registry from CorEvitas, part of the PPD clinical research business of Thermo Fisher Scientific.
It complements an existing CorEvitas registry focused on AA in adults, launched in 2023. The adolescent AA registry was launched under the scientific guidance of Britt Craiglow, MD, Associate Professor, Adjunct of Dermatology, Yale School of Medicine in New Haven, CT; Maryanne Senna, MD, Assistant Professor of Dermatology at Harvard Medical School in Boston, MA; and Brett King, MD, PhD, of Dermatology Physicians of Connecticut, as scientific advisors to the registry.
The registry is designed to prospectively study alopecia areata, including the incidence of safety events of interest, medication utilization patterns and the comparative safety and effectiveness of treatments. It will also gather data on the history of AA in adolescents and the growth and development over time of adolescent AA patients undergoing treatment with new therapies.
To learn more about this study and the newly launched registry, request information at alopecia@corevitas.com (U.S. and Canada) or alopecia.adol@corevitas.com (EU).
PHOTO CREDIT: DermNet