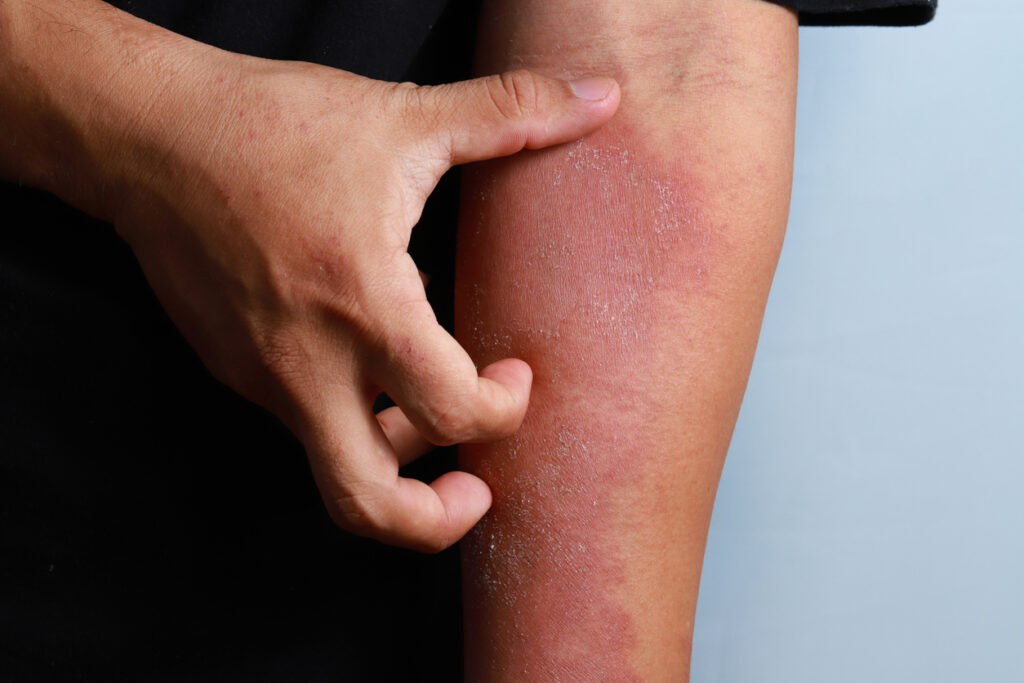
Celldex Therapeutics, Inc. has initiated a Phase 2 study of barzolvolimab in atopic dermatitis (AD).
Barzolvolimab is a humanized monoclonal antibody that specifically binds and blocks the receptor tyrosine kinase KIT with high specificity and potently inhibits its activity, which is required for the function and survival of the mast cell.
“Two-thirds of patients treated with first line systemic therapy do not achieve complete control of their atopic dermatitis and desperately need new therapies that offer rapid, meaningful relief from the severe itching and breakdown of the skin associated with AD,” says Diane C. Young, M.D, Senior Vice President and Chief Medical Officer of Celldex Therapeutics, in a news release. “We believe mast cells play a critical role in the pathophysiology of AD and utilizing our mast cell depleting agent, barzolvolimab, could yield meaningful benefit for patients by helping them resolve their disease and reclaim their quality of life. We’re pleased to end this year with the advancement of barzolvolimab into its fifth indication and look forward to an exciting year ahead in 2025 with multiple upcoming data readouts.”
The randomized, double-blind, placebo-controlled Phase 2 study is evaluating the efficacy and safety profile of subcutaneous barzolvolimab in patients with moderate to severe AD. Approximately 120 patients will be randomly assigned on a 1:1:1 ratio to receive subcutaneous injections of barzolvolimab at either 150 or 300mg or placebo every four weeks after an initial loading dose of 450mg or placebo during a 16-week placebo-controlled treatment phase. Participants randomized into the placebo arm will be re-randomized at Week 16 into one of the two active treatment arms. Patients then enter a 16-week active treatment phase, in which all patients will receive barzolvolimab every four weeks. The primary endpoint of the study is to evaluate the clinical efficacy of the two dose levels compared to placebo using the Peak Pruritus Numerical Rating Scale (PP-NRS) at Week 16, a well-defined, reliable, sensitive and valid scale for evaluating worst itch intensity in adults with moderate ton severe AD. Secondary endpoints include the evaluation of the clinical efficacy of barzolvolimab, compared to placebo across multiple patient-reported outcomes, including assessing impressions of disease change and severity and improvements in quality of life.
The study is now actively enrolling patients. When all clinical trial sites are open, the study will include up to 50 clinical trial centers in the United States.