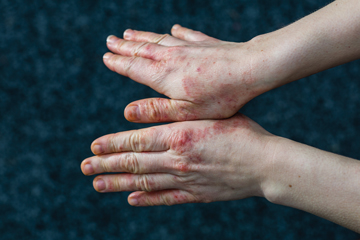
The Medicines and Healthcare Products Regulatory Agency (MHRA) has granted marketing authorization in Great Britain for delgocitinib cream (Anzupgo, Leo Pharma) for the treatment of adult patients with moderate to severe chronic hand eczema (CHE), for whom topical corticosteroids are inadequate or inappropriate.
Delgocitinib cream is now the first approved topical pan-Janus kinase (JAK) inhibitor treatment specifically for adults with moderate to severe CHE in Great Britain.
“Today’s MHRA approval of delgocitinib cream marks a significant milestone for adults in Great Britain living with moderate to severe Chronic Hand Eczema,” says Leanne Walsh, Vice President and General Manager, LEO Pharma UK and Ireland, in a news release. “This approval offers a new treatment paradigm and demonstrates our commitment to addressing the unmet needs of people living with skin conditions.”
The MHRA decision was based on results from the delgocitinib cream Phase 3 program, which included the DELTA 1 and DELTA 2 clinical trials that evaluated the safety and efficacy of delgocitinib cream compared to the cream vehicle. Both trials met their primary and all secondary endpoints. Subjects who completed the 16-week DELTA 1 or DELTA 2 trials were immediately offered to enroll in the 36-week DELTA 3 open-label extension trial.
LEO Pharma UK and Ireland is collaborating closely with the National Institute for Health and Care Excellence (NICE) to make delgocitinib cream available on the National Health Service (NHS).