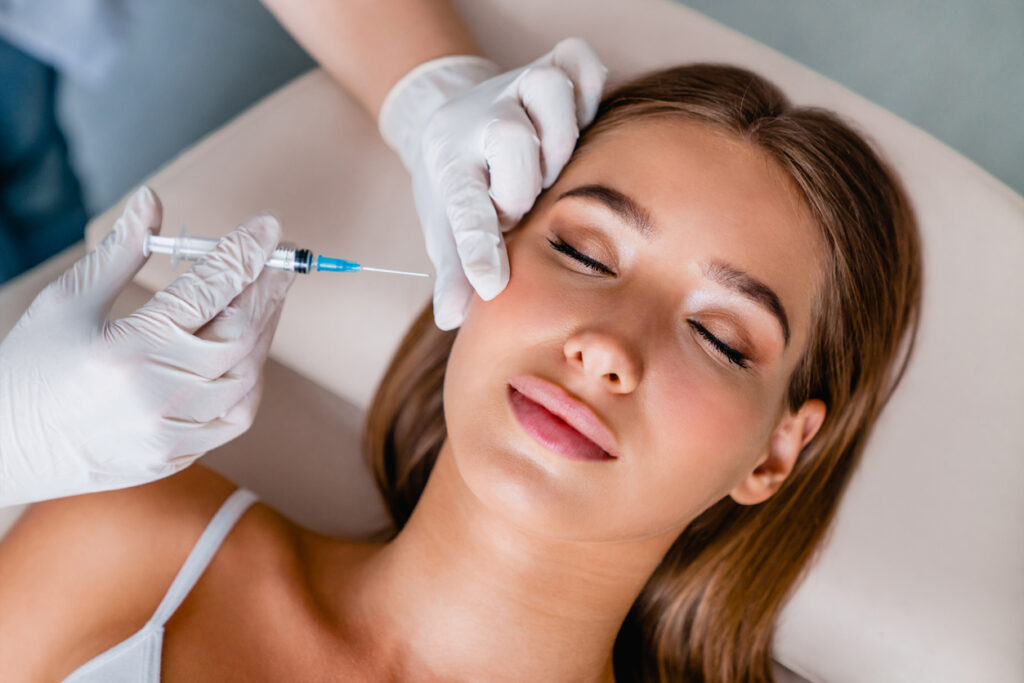
The EU Medical Device Regulation (MDR) certification gave its nod to four injectable hyaluronic acid (HA) gel fillers from Evolus under the brand name Estyme,
The CE Mark certification, through the new MDR process, represents more stringent requirements, designed to ensure the safety, efficacy, and quality of medical devices sold in Europe.
Evolus will introduce Estyme through a limited experience program with select physician partners. A broader European launch is planned for the second half of 2025.
“The MDR CE Mark certification for Estyme , is a critical milestone in our strategic path to expand our product portfolio and reflects our commitment to delivering high-quality gels that adhere to the most rigorous regulatory standards,” says David Moatazedi, President and Chief Executive Officer of Evolus, in a news release.. “With Estyme , we are bringing a new level of excellence to the dermal filler category, as we grow our product portfolio to meet growing consumer demand for premium, cash-pay aesthetic treatments.”
These fillers are branded as Evolysse in the U.S. market. The company remains on track for the U.S. approval and launch of the Evolysse injectable HA gels beginning in 2025.