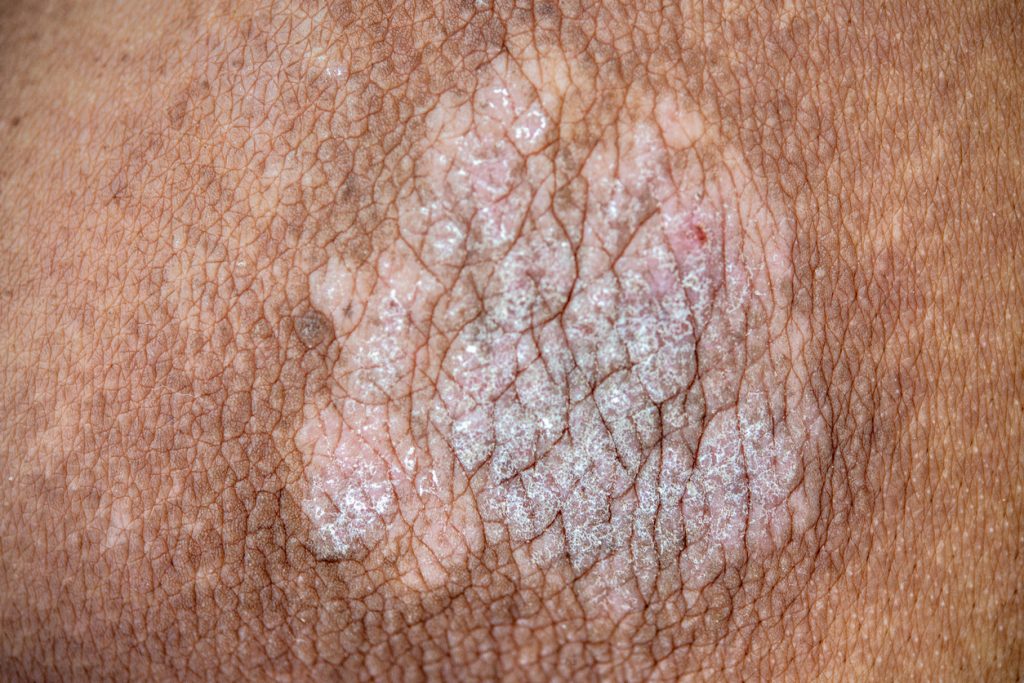
Johnson & Johnson is offering health care providers access to the TREMFYA Clearance Photo Library, a longitudinal library of more than 20,000 before and after psoriasis treatment photographs from the Phase 3b VISIBLE study.
The online resource enables patient images to be filtered based on clinical characteristics, such as disease severity, areas of involvement, and clearance outcomes across all skin tones.
Currently, only 4-19% of images in dermatology textbooks show PsO on darker skin tones, which makes it challenging to recognize the disease in patients with skin of color. People of color living with plaque and scalp PsO also face challenges such as misdiagnosis and delayed diagnosis at much higher rates than white individuals with PsO. The TREMFYA Clearance Photo Library will continue to be updated with additional images from ongoing and future studies that address underrepresented PsO patient types, with the goal of setting a new standard for inclusion in medical research.
VISIBLE is a Phase 3b, multicenter, randomized, double-blind, placebo-controlled (Weeks 0-16) trial in adult patients (≥18 years of age) with moderate to severe body and/or scalp PsO. Patients (n=211) were randomized to guselkumab (Tremfya) 100mg subcutaneous injection at Weeks 0, 4, and 12, then q8w; placebo at Weeks 0, 4, and 12, followed by crossover to guselkumab at Week 16, Week 20, and q8w. The study was designed to evaluate the efficacy and safety of guselkumab in skin of color patients (self-identify as non-white) across the entire spectrum of the Fitzpatrick scale (I-VI).