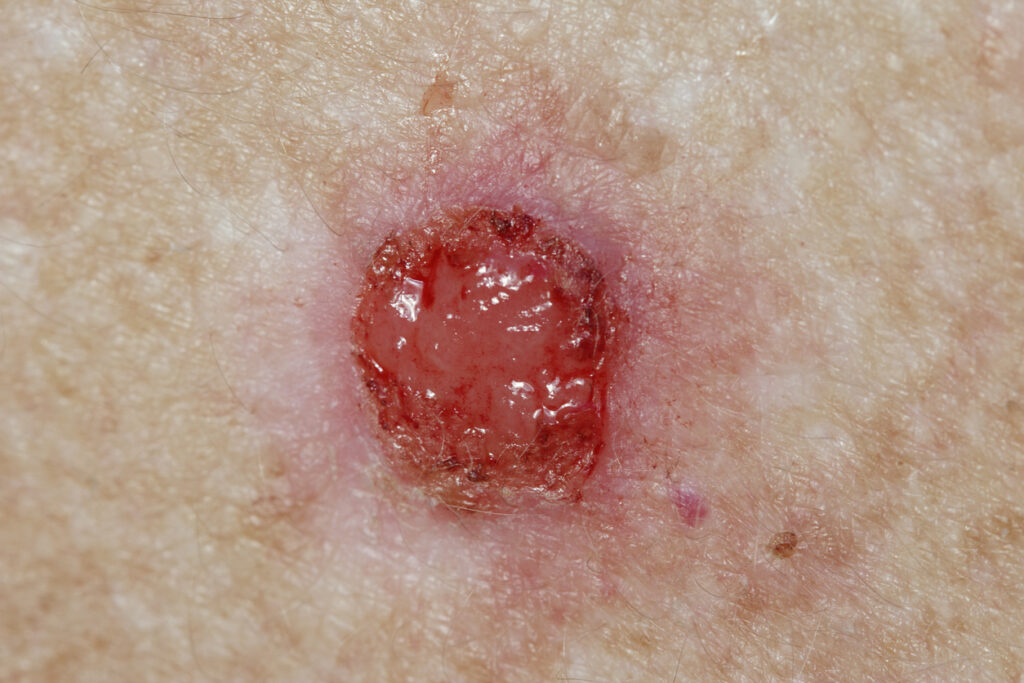
It’s a (database) lock for Biofrontera Inc.’s Phase 3 study of Ameluz–photodynamic therapy (PDT) in combination with the BF-RhodoLED lamp in patients with superficial basal cell carcinoma (sBCC).
A database lock finalizes the data collected and prevents further changes to the data. It’s usually one of the last steps before submitting the data to the FDA. The data are now being analyzed with interim results expected in November 2024.
This Phase 3 ALA-BCC-CT013 trial is a double-blind, randomized, placebo-controlled multi-center study involving 186 patients with one or more clinically and histologically confirmed superficial BCCs. They each received one cycle of two PDT treatments (either Ameluz-PDT or placebo-PDT) 1-2 weeks apart, repeated after three months if required. The primary endpoint is the composite complete clinical and histological clearance of the target BCC lesion 12 weeks after the start of the last PDT cycle. Secondary efficacy parameters and drug safety were evaluated.
In addition to the final study report, the U.S. Food and Drug Administration (FDA) requires the inclusion in the submission of follow-up data obtained one year after the first PDT. The last patient is expected to complete this follow up by December of this year, and submission is targeted for Q1 2025.
“If this indication is granted by the FDA this would expand our label from premalignant application for Actinic Keratoses to the treatment of cutaneous malignancy. It would be the next stage in our continued development of PDT and part of our vision to become the market leaders in this field” says Dr Hermann Luebbert, CEO and Chairman of Biofrontera Inc., in a news release.