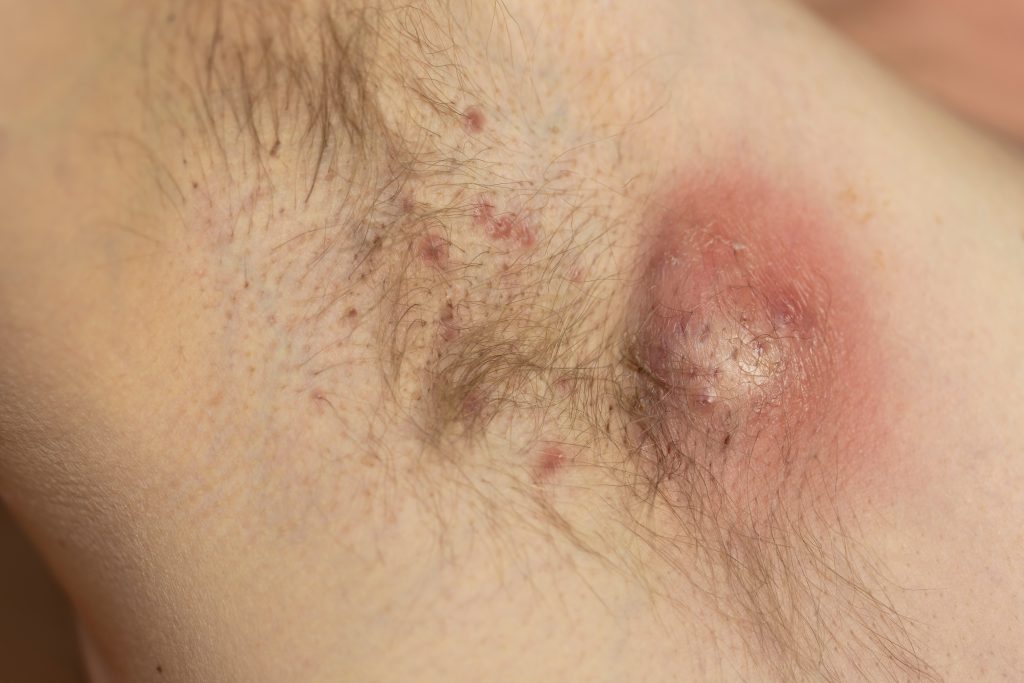
The first patient has been dosed in Avalo Therapeutics, Inc.’s Phase 2 LOTUS trial of AVTX-009 in hidradenitis suppurativa (HS).
AVTX-009 is a humanized monoclonal antibody (IgG4) that binds to interleukin-1β (IL-1β) with high affinity and neutralizes its activity.
The LOTUS Trial is a randomized, double-blind, placebo-controlled, parallel-group Phase 2 trial with two AVTX-009 dose regimens to evaluate the efficacy and safety of AVTX-009 in approximately 180 adults with moderate to severe hidradenitis suppurativa. Subjects will be randomized (1:1:1) to receive either one of two doses of AVTX-009 or placebo during a 16-week treatment phase. The primary efficacy endpoint is the proportion of subjects achieving Hidradenitis Suppurativa Clinical Response (HiSCR75) at Week 16. Secondary objectives include but are not limited to: proportion of patients achieving HiSCR50 and HiSCR90 as well as change from baseline in: International HS Severity Score System (IHS4), draining fistula count, abscess and inflammatory nodule (AN) count and patients achieving at least a 30% reduction on a numerical rating scale in Patient’s Global Assessment of Skin Pain (PGA Skin Pain). The number of patients with anti-drug antibodies, safety, and tolerability will be assessed.
Topline results are expected in 2026.
For additional information this trial (NCT06603077), please visit www.clinicaltrials.gov.