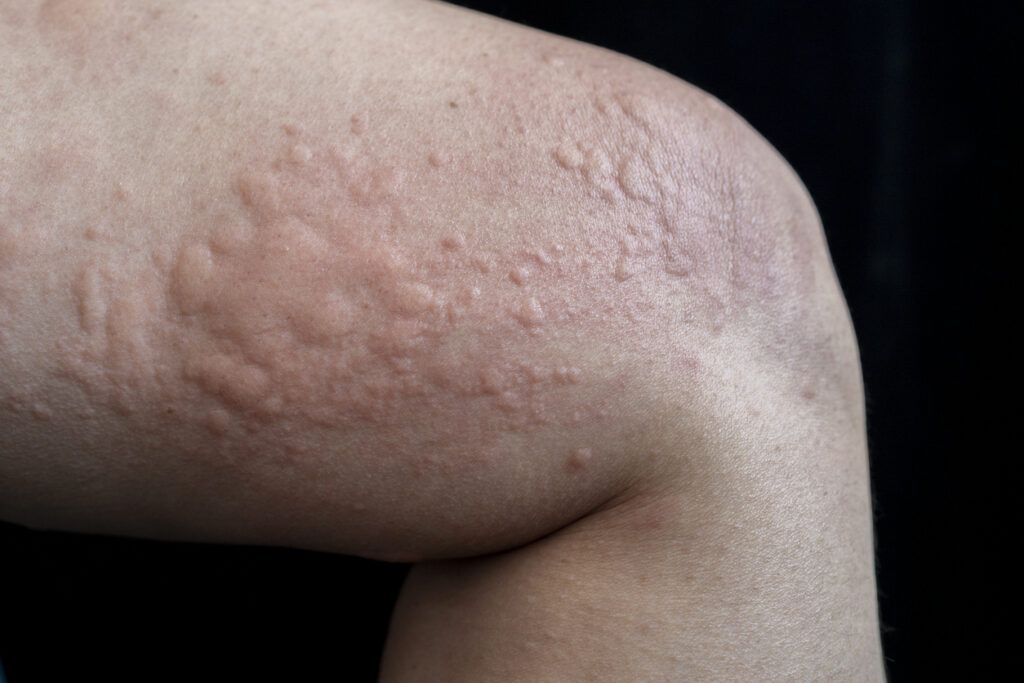
The first patient has been enrolled in a Phase 2 trial of Evommune, Inc.’s EVO756 in adults with chronic inducible urticaria (CIndU).
The trial aims to evaluate EVO756 in approximately 30 patients with symptomatic dermographism and cold urticaria in 15 study sites across the United States. EVO756 is a potent, highly selective small molecule antagonist of mas-related G-protein coupled receptor X2 (MRGPRX2). It holds the potential for once-daily oral administration without the serious side effects observed with other therapies.
Efficacy endpoints include changes from baseline in disease specific provocation thresholds that are used as objective markers to quantify disease severity and response to treatment. EVO756 will be administered orally, once daily, for four weeks, and will be evaluated for safety and efficacy at weekly visits during treatment, with patients serving as their own control.
“Initiation of this trial marks another important milestone in dedication to delivering the therapeutic potential of MRGPRX2 antagonism to patients in a broad range of mast cell-mediated disorders,” says Daniel J. Burge, MD, Senior Vice President, Clinical Development at Evommune, in a news release. “Following the success of our proof-of-concept study with EVO756 earlier this year, we are also on track to initiate a Phase 2b trial in chronic spontaneous urticaria in the second quarter of 2025.”