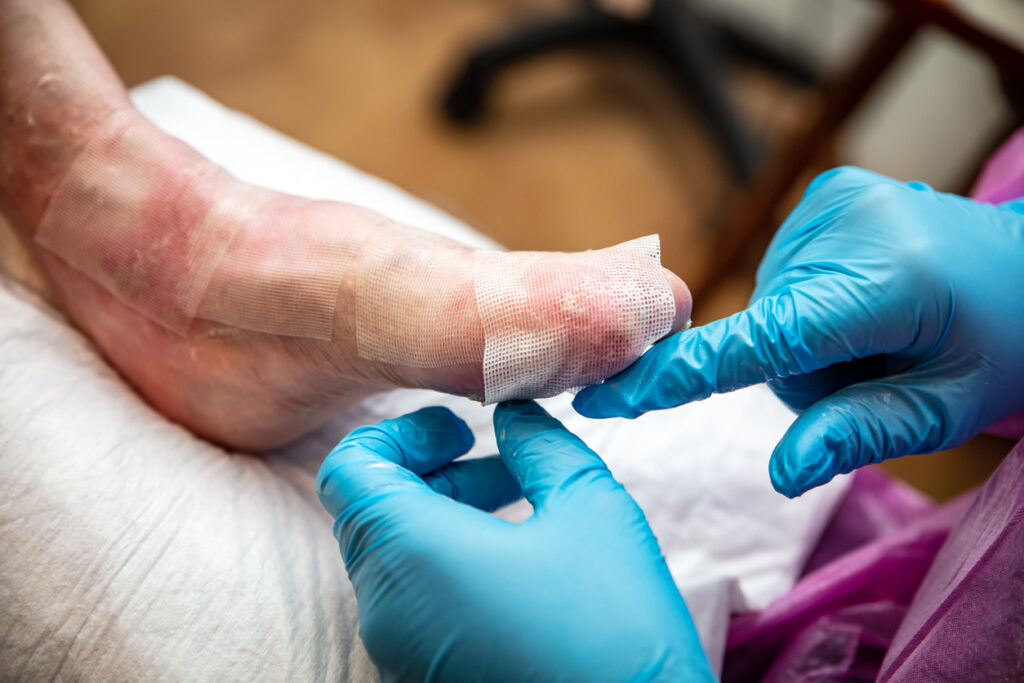
The first patient has been dosed in a Phase 1/2a clinical study evaluating AGLE-102 for the treatment of dystrophic epidermolysis bullosa (DEB), Aegle Therapeutics Corp. reports.
AGLE-102 is an investigational product comprised of extracellular vesicles isolated from allogeneic stem cells using Aegle’s proprietary methods. AGLE-102 is a native composite of cell-derived nanoparticles that contain active biomolecules including proteins and nucleic acids with anti-inflammatory, immunomodulating, and tissue regenerating properties.
“AGLE-102 is designed to mimic the body’s healing mechanism by delivering proteins, including collagen 7, and other important biomolecules such as nucleic acids that act by reducing inflammation, modulating the immune system and inducing diseased cells to make their own collagen 7,” explains Evangelos Badiavas, MD, PhD, Co-Founder and Chief Scientific Officer, in a news release. “AGLE-102 offers a unique, multifaceted approach to the treatment of DEB.”
The phase I/2a trial, “A Safety Study of the Administration of Mesenchymal Stem Cell Extracellular Vesicles in the Treatment of Dystrophic Epidermolysis Bullosa Wounds”, is a prospective, non-randomized, multi-center study designed to evaluate the safety and efficacy of multiple doses of AGLE-102 in the treatment of chronic lesions.
AGLE-102 is being evaluated in two phase 1/2a studies in DEB and severe burns. AGLE-102 is also in a preclinical program for GvHD.
More information on the study (NCT04173650) is available at clinicaltrials.gov.