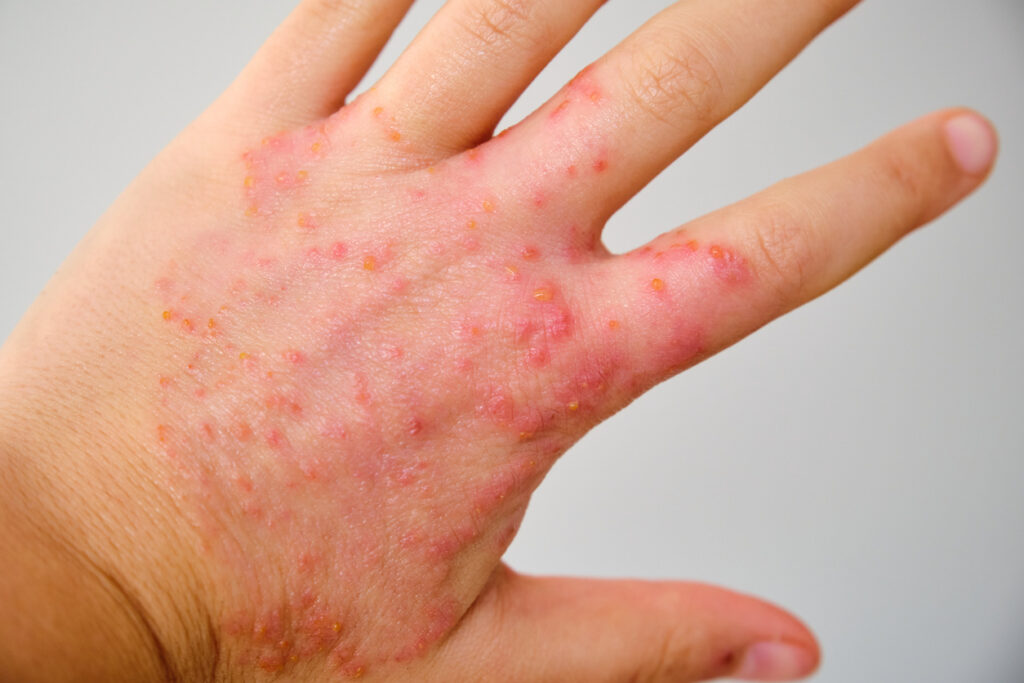
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommends the approval of Leo’s delgocitinib cream (Anzupgo) for the treatment of adult patients with moderate to severe chronic hand eczema (CHE), for whom topical corticosteroids are inadequate or inappropriate.
The positive opinion marks the latest step toward marketing authorization in the European Union (EU), with a final decision from the European Commission (EC) now pending. If approved, delgocitinib cream will be the first topical treatment specifically indicated for adults with moderate to severe CHE for whom topical corticosteroids are inadequate or inappropriate.
The CHMP’s positive opinion will be reviewed by the European Commission (EC) and, pending the final decision, authorization of delgocitinib cream will be valid in all EU member states, Iceland, Norway, and Liechtenstein. Regulatory filings in other markets are underway.
Delgocitinib cream is an investigational topical pan-JAK inhibitor for CHE. It inhibits the activation of JAK-STAT signaling, which plays a key role in the pathogenesis of CHE. Currently, there are no topical treatments specifically approved for adults with moderate to severe CHE for whom topical corticosteroids are inadequate or inappropriate.
The positive CHMP opinion for delgocitinib cream is based on results from the phase 3 program, which includes the DELTA 1 and DELTA 2 clinical trials that evaluated the safety and efficacy of delgocitinib cream compared to cream vehicle. Both trials met their primary and all secondary endpoints. Subjects who completed the 16-week DELTA 1 or DELTA 2 trials were immediately offered to enroll in the 36-week DELTA 3 open-label extension trial. Results demonstrated a long-term safety profile consistent with previous trial results.
About the DELTA 1, 2 and 3 Trials
The primary objective for the randomized, double-blind, vehicle-controlled, multi-center phase 3 clinical trials (DELTA 1 and DELTA 2) was to evaluate the efficacy of twice-daily applications of delgocitinib cream compared with cream vehicle in the treatment of adults with moderate to severe CHE.
The primary endpoint of the trials was the Investigator’s Global Assessment for chronic hand eczema treatment success (IGA-CHE TS) at Week 16. Treatment success was defined as an IGA-CHE score of 0 (clear) or 1 (almost clear) with at least a two-step improvement from baseline. Additional IGA-CHE scores included 2 (mild), 3 (moderate), and 4 (severe).
Key secondary endpoints at Week 16 included reduction of itch and pain scores of ≥4 points measured by the Hand Eczema Symptom Diary (HESD) from baseline to Week 16, as well as at least 75% improvement from baseline and at least 90% improvement from baseline on the Hand Eczema Severity Index (HECSI) at Week 16. The number of treatment-emergent adverse events from baseline to Week 16 defined the key safety endpoint of the trials.
Subjects who completed 16 weeks of treatment with delgocitinib cream or cream vehicle twice daily in trials DELTA 1 or DELTA 2 were offered to roll-over to the 36-week DELTA 3 Open-label, Multi-site Extension trial. The purpose of this extension trial was to evaluate the long-term safety of delgocitinib.