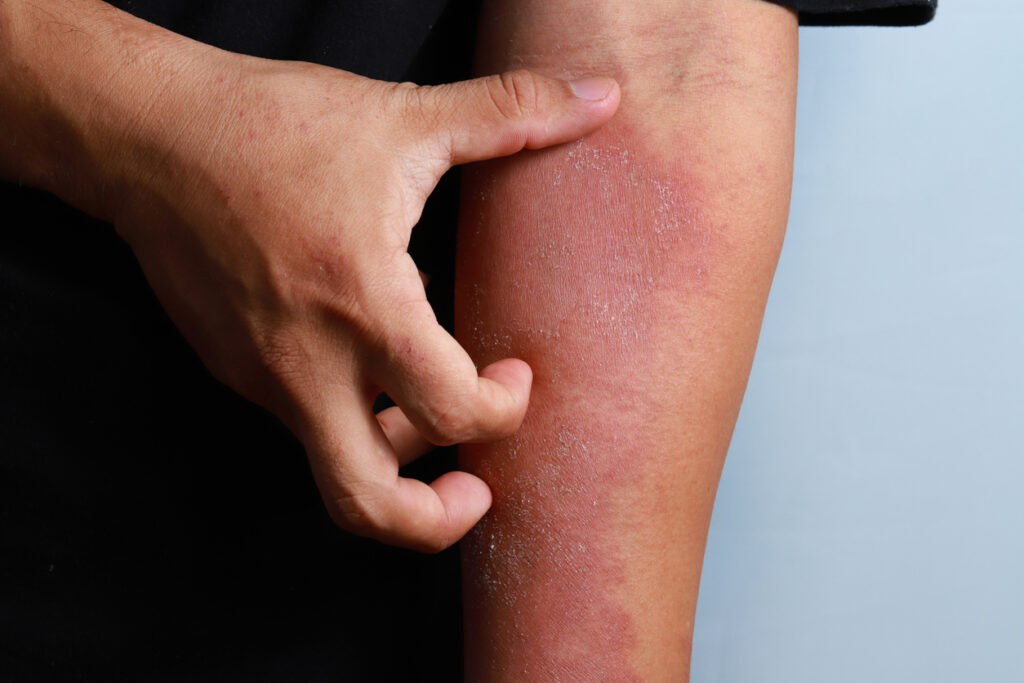
Limiting the use of oral corticosteroid treatment to 90 days or less may limit adverse effects in patients with atopic dermatitis (AD).
This is the main takeaway from a new nested case-control study in JAMA Network Open that looked at the long-term use of oral corticosteroids and safety outcomes for AD patients.
Specifically, the use of oral corticosteroids for more than 90 days during one year was associated with a slightly increased risk of composite adverse outcomes: osteoporosis, fracture, type 2 diabetes, hyperlipidemia, hypertension, myocardial infarction, stroke, heart failure, avascular necrosis, cataract, or glaucoma.
There was no increased risk with use of oral corticosteroids for more than 30 days, the study showed.
“Future investigations are warranted to confirm this potential risk of adverse events (AEs) associated with long-term use of oral corticosteroids for patients with exacerbations of atopic dermatitis, and health care professionals should thoroughly weigh the benefits associated with oral corticosteroids against the observed small risk of AEs, while continuously monitoring for AEs,” the study authors conclude.