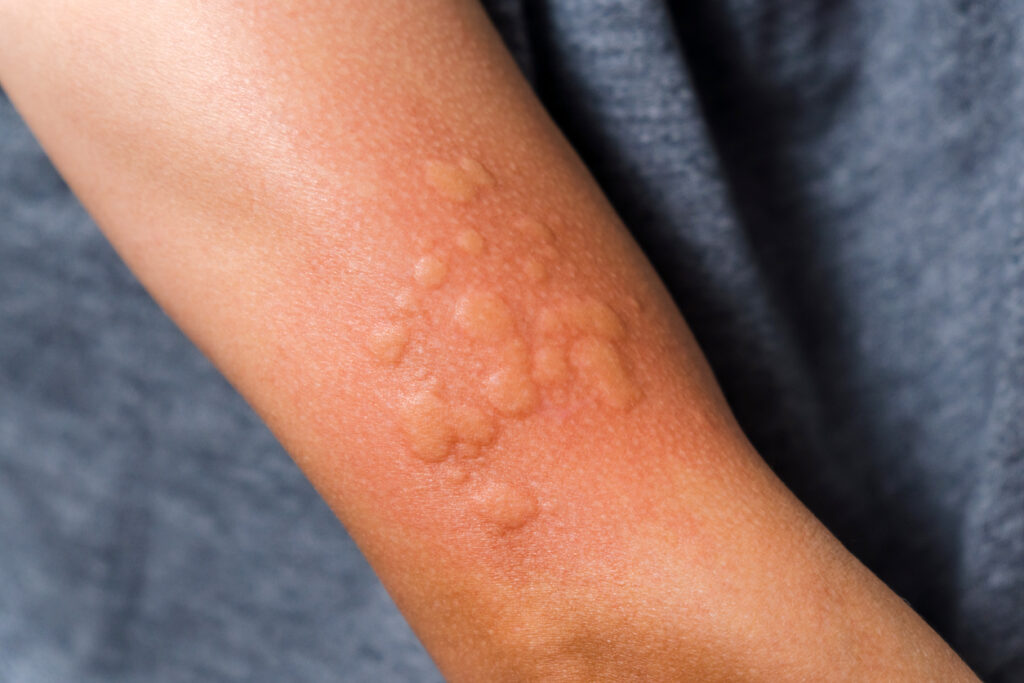
Celldex Therapeutics, Inc. is starting its global Phase 3 program, consisting of two Phase 3 trials (EMBARQ-CSU1 and EMBARQ-CSU2) designed to establish the efficacy and safety of barzolvolimab in adult patients with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment.
The studies will also include patients who remain symptomatic after treatment with biologics.
Barzolvolimab, a novel monoclonal antibody, works upstream of other treatment approaches for CSU, targeting mast cells by blocking the receptor tyrosine kinase KIT, which is required for mast cell function and survival.Barzolvolimab is also currently being studied in chronic inducible urticaria (CIndU), prurigo nodularis (PN) and eosinophilic esophagitis (EOE) with additional indications planned for the future, including atopic dermatitis (AD).
“Across multiple studies in CSU, barzolvolimab has demonstrated a unique and profound ability to offer rapid, durable and complete disease control, regardless of prior treatment history, to patients suffering from this often severe and debilitating disease,” says Marcus Maurer, MD, Professor of Dermatology and Allergy at Charité – Universitätsmedizin in Berlin, Germany and a Principal Investigator in the Phase 3 studies of Barzolvolimab in a news release. “Advancing a promising agent that addresses the root driver of the disease—the mast cell—into late stage studies is a significant and hopeful step forward for patients and physicians who desperately need better treatment options.”
EMBARQ-CSU1 and EMBARQ-CSU2 are designed to establish the efficacy and safety of barzolvolimab in adult patients with CSU who remain symptomatic despite H1 antihistamine treatment. Both Phase 3 trials are randomized, double-blind, placebo-controlled, parallel group, global studies where approximately 915 patients will be randomized evenly to barzolvolimab 150mg every 4 weeks (following 300mg loading dose), barzolvolimab 300mg every 8 weeks (following 450mg loading dose) or placebo for 52 weeks. At 24 weeks, patients on placebo will be re-randomized to active treatment across both dosing groups. The primary endpoint of the study will evaluate the clinical effect of barzolvolimab in reducing urticaria activity (weekly urticaria activity score; UAS7) at Week 12. The study is designed to detect a clinically meaningful difference between each of the active arms vs placebo in the overall population as well as in the subpopulation of omalizumab refractory participants.
Results from a placebo-controlled Phase 2 study in CSU demonstrated that barzolvolimab met the primary endpoint of the study, a statistically significant mean change from baseline to week 12 in UAS7, across all dose levels. Secondary and exploratory endpoints in the study were also achieved at week 12 and strongly support the primary endpoint results, including changes in ISS7 (weekly itch severity score) and HSS7 (weekly hives severity score) and responder analyses (UAS7<=6 or UAS7=0). Importantly, barzolvolimab demonstrated rapid, durable and clinically meaningful responses in patients with moderate to severe CSU refractory to antihistamines, including patients with prior omalizumab treatment and was well tolerated with a favorable safety profile.