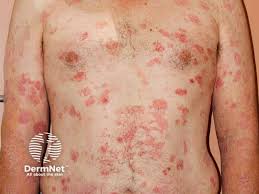
Extended HyBryte treatment for up to 12 months is showing promise in patients with early-stage cutaneous T-cell lymphoma (CTCL), according to an interim update from an open-label, investigator-initiated study (IIS).
Soligenix, Inc.’s HyBryte (research name SGX301) is a novel, first-in-class photodynamic therapy that utilizes safe, visible light for activation. The active ingredient in HyBryte is synthetic hypericin, a potent photosensitizer that is topically applied to skin lesions, taken up by the malignant T-cells, and then activated by safe, visible light approximately 24 hours later.
The use of visible light in the red-yellow spectrum can penetrate more deeply into the skin (much more so than ultraviolet light) and therefore potentially treating deeper skin disease and thicker plaques and lesions.
This treatment approach avoids the risk of secondary malignancies (including melanoma) inherent with the frequently employed DNA-damaging drugs and other phototherapy that are dependent on ultraviolet exposure.
HyBryte has received orphan drug and fast-track designations from the U.S. Food and Drug Administration (FDA) and orphan designation from the European Medicines Agency (EMA). In addition, the FDA awarded an Orphan Products Development grant to support the evaluation of HyBryte for expanded treatment in patients with early-stage CTCL, including in the home use setting. The grant, totaling $2.6 million over four years, was awarded to the University of Pennsylvania, a leading enroller in the Phase 3 FLASH (Fluorescent Light Activated Synthetic Hypericin) study.
The initial Phase 3 FLASH study demonstrated the safety and efficacy of shorter courses of HyBryte therapy. Now, continuing treatment for longer time periods is resulting in anticipated improved patient outcomes.
The new clinical study RW-HPN-MF-01, “Assessment of Treatment with Visible Light Activated Synthetic Hypericin Ointment in Mycosis Fungoides Patients,” is designed as an open-label, multicenter clinical trial enrolling up to 50 patients at select U.S. clinical centers. Patients have the potential to be treated for up to 12 months with twice-a-week dosing (visible light activation following ointment application by 24 ± 6 hours). The study also allows for a potential transition to a “real-world” setting with home use. The primary endpoint for the study is evaluating the number of treatment successes defined as ≥50% reduction in the cumulative mCAILS score from the Baseline to the end of the treatment.
To date, six patients have been enrolled and treated with HyBryte over a time period ranging up to 44 weeks. Patients have responded positively to HyBryte therapy, with 75% (three of the four subjects who have completed at least 12 weeks of therapy) already achieving “treatment success” as predefined in the study’s protocol as ≥50% improvement in their cumulative modified Composite Assessment of Index Lesion Severity (mCAILS) score compared to Baseline. Two of the three treatment successes were achieved within the first 12 weeks, and the third within 18 weeks. Two of the remaining three patients have only recently started HyBryte therapy and have not yet reached their first efficacy evaluation visit (i.e., at Week 6). The other had a substantial improvement documented at the Week 18 visit but has not achieved the success threshold yet. In addition, HyBryte appears to be safe and well tolerated in all patients, with no treatment-related adverse events reported to date.
“In the Phase 3 FLASH study, HyBryte was shown to be productive with a promising safety profile. With limited treatment options, especially in the early stages of their disease, CTCL patients are often searching for alternative treatments. In our U.S. Food and Drug Administration (FDA)-funded study, initial results evaluating the expanded use of HyBryte in a ‘real world’ treatment setting are promising, further supporting and extending results from the previous positive Phase 2 and 3 clinical trials,” notes Ellen Kim, MD, Director, Penn Cutaneous Lymphoma Program, Vice Chair of Clinical Operations, Dermatology Department, and Professor of Dermatology at the Hospital of the University of Pennsylvania in Philadelphia, PA, in a news release. She was a leading enroller in the Phase 3 FLASH (Fluorescent Light Activated Synthetic Hypericin) study for treating early-stage CTCL. “We look forward to continuing to work with the FDA to complete this study and participating in the upcoming confirmatory Phase 3 placebo-controlled study.”
The published Phase 3 FLASH trial enrolled 169 patients (166 evaluable) with Stage IA, IB, or IIA CTCL. The trial consisted of three treatment cycles. Treatments were administered twice weekly for the first six weeks, and treatment response was determined at the end of the 8th week of each cycle. In the first double-blind treatment cycle (Cycle 1), 116 patients received HyBryte treatment (0.25% synthetic hypericin), and 50 received placebo treatment of their index lesions. A total of 16% of the patients receiving HyBryte achieved at least a 50% reduction in their lesions (graded using a standard measurement of dermatologic lesions, the CAILS score) compared to only 4% of patients in the placebo group at eight weeks during the first treatment cycle (primary endpoint). HyBryte treatment in this cycle was safe and well tolerated.
In the second open-label treatment cycle (Cycle 2), all patients received HyBryte treatment of their index lesions. Evaluation of 155 patients in this cycle (110 receiving 12 weeks of HyBryte treatment and 45 receiving six weeks of placebo treatment followed by six weeks of HyBryte treatment) demonstrated that the response rate among the 12-week treatment group was 40%. Comparison of the 12-week and 6-week treatment responses also revealed a statistically significant improvement between the two-time points, indicating that continued treatment results in better outcomes. HyBryte continued to be safe and well-tolerated. Additional analyses also showed that HyBryte is equally effective in treating both plaque and patch lesions of CTCL, a particularly relevant finding given the historical difficulty in treating plaque lesions.
The third (optional) treatment cycle (Cycle 3) was focused on safety, and all patients could elect to receive HyBryte treatment for all their lesions. Of note, 66% of patients elected to continue with this optional compassionate use/safety cycle of the study. Of the subset of patients that received HyBryte throughout all three cycles of treatment, 49% of them demonstrated a positive treatment response. Moreover, a subset of patients evaluated in this cycle demonstrated that HyBryte is not systemically available, consistent with the general safety of this topical product observed to date. At the end of Cycle 3, HyBryte continued to be well tolerated despite extended and increased use of the product to treat multiple lesions.
Overall safety was monitored throughout the three treatment cycles (Cycles 1, 2, and 3) and the 6-month follow-up period. HyBryte’s mechanism of action is not associated with DNA damage, making it a safer alternative than currently available therapies, all of which are associated with significant and sometimes fatal side effects.
Following the first Phase 3 study of HyBryte for the treatment of CTCL, the FDA, and the EMA indicated that they would require a second successful Phase 3 trial to support marketing approval.
With agreement from the EMA on the key design components, the second confirmatory study, called FLASH2, is expected to be initiated before the end of 2024. This study is a randomized, double-blind, placebo-controlled, multicenter study that will enroll approximately 80 subjects with early-stage CTCL. The FLASH2 study replicates the double-blind, placebo-controlled design used in the first successful Phase 3 FLASH study that consisted of three 6-week treatment cycles (18 weeks total), with the primary efficacy assessment occurring at the end of the initial 6-week double-blind, placebo-controlled treatment cycle (Cycle 1). However, this second study extends the double-blind, placebo-controlled assessment to 18 weeks of continuous treatment (no “between-cycle” treatment breaks), with the primary endpoint assessment occurring at the end of the 18-week timepoint. In the first Phase 3 study, a treatment response of 49% was observed in patients completing 18 weeks (3 cycles) of therapy. In this second study, all important clinical study design components remain the same as in the first FLASH study, including the primary endpoint and key inclusion-exclusion criteria. The extended treatment for a continuous 18 weeks in a single cycle is expected to statistically demonstrate HyBryte’s increased effect over a more prolonged, “real world” treatment course.
PHOTO CREDIT: DermNet