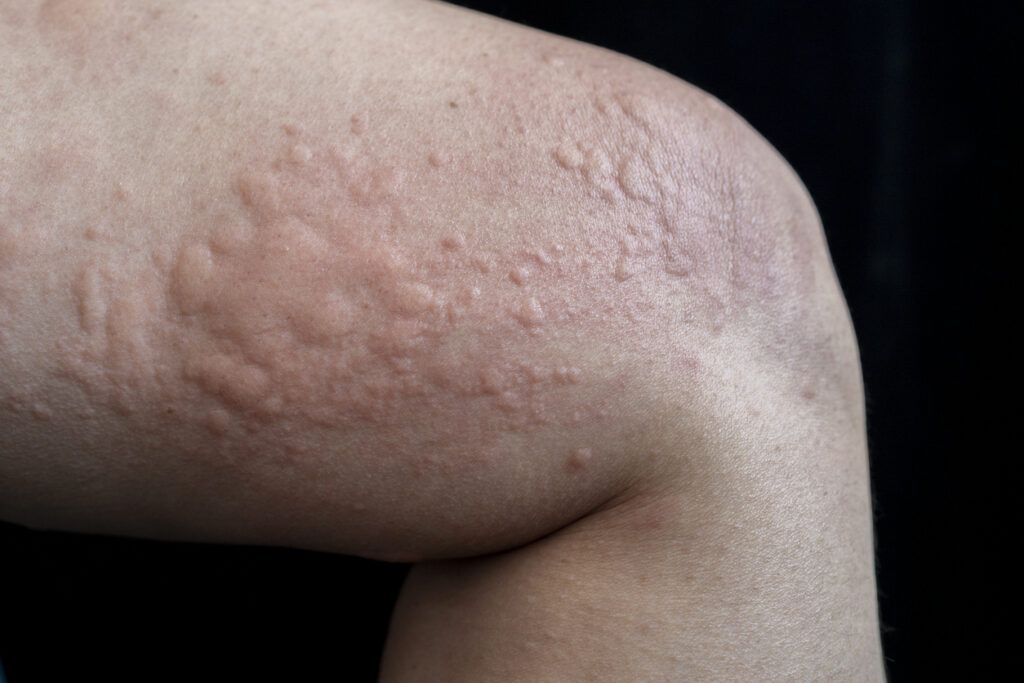
Remibrutinib (Novartis) shows sustained efficacy and long-term safety in chronic spontaneous urticaria (CSU), according to data from two Phase III studies.
Remibrutinib is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor being studied in chronic spontaneous urticaria (CSU) and other immune-mediated conditions such as hidradenitis suppurativa. Novartis will submit remibrutinib for approval in CSU to global health authorities starting in H2 2024.
In the pivotal Phase III studies, REMIX-1 and REMIX-2, remibrutinib treatment showed significant symptom improvement early, which was sustained up to Week 52, in patients with CSU who remained symptomatic despite second-generation H1-antihistamine use.
These data are being presented at the 2024 European Academy of Allergy and Clinical Immunology (EAACI) Congress in Valencia, Spain, May 31–June 3.
“These data show that remibrutinib has the potential to offer patients and physicians a well-tolerated oral treatment that provides early and lasting efficacy,” says Martin Metz, Professor of Dermatology, Charité – Universitätsmedizin in Berlin, Germany, in a news release.
The Week 52 data show significant improvements with remibrutinib versus placebo, as previously shown at Week 12, were confirmed at Week 24, including in weekly urticaria activity score (UAS7), weekly itch severity score (ISS7), and weekly hive severity score (HSS7).
At Week 24, patients receiving placebo were transitioned to remibrutinib, and responses with remibrutinib were observed as early as the first week after switching and were sustained until the end of the study (28 weeks of treatment). Almost half of patients were completely free of itch and hives (UAS7=0) as assessed at Week 52, the study showed,
Remibrutinib was well-tolerated and demonstrated a favorable and consistent safety profile up to 52 weeks, including balanced liver function tests versus placebo. Adverse events (AEs), including serious AEs and treatment discontinuations due to AEs, were comparable between remibrutinib and placebo during the 24-week placebo-controlled period. In addition, exposure-adjusted rates did not increase with long-term treatment. Liver transaminase elevations were balanced across the remibrutinib and placebo treatment groups; all were asymptomatic, transient, and reversible1. None of the serious AEs were considered related to study medication by investigators.
“The 52-week REMIX-1 and REMIX-2 Phase III data are significant as many patients who had moderate to severe urticaria at study start were completely free of itch and hives after 52 weeks of treatment and remibrutinib, a highly selective oral BTK inhibitor, continued to be well tolerated,” says said Angelika Jahreis, Global Head, Development, Immunology, Novartis.