AbbVie Submits BLA to FDA for Rapid Onset Glabellar Lines Treatment
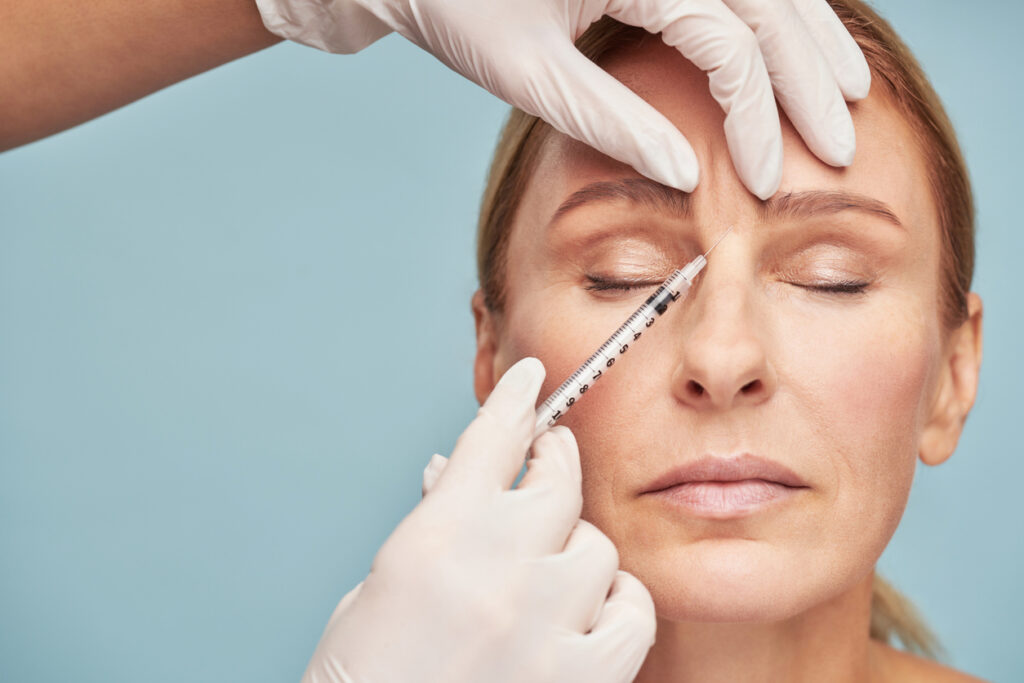
AbbVie has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for trenibotulinumtoxinE (TrenibotE) for the treatment of moderate to severe glabellar lines.
