Enrollment Complete for Phase 3 Trial of Candin for the Treatment of Common Warts
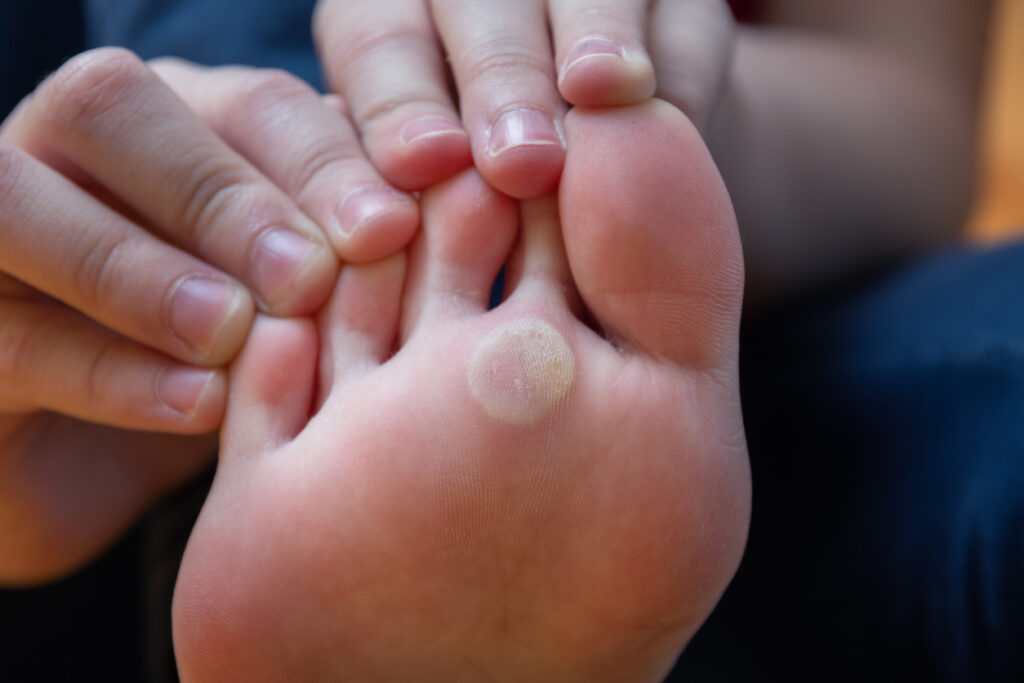
The final patient has been successfully enrolled in Nielsen BioSciences, Inc.’s CFW-3A, a Phase 3, randomized, double-blind, placebo-controlled study of the safety and rfficacy of Candin (Candida albicans Skin Test Antigen for Cellular Hypersensitivity) for the treatment of common warts (Verruca vulgaris) in adolescents and adults. Candin is currently marketed in the United States for […]
