U.S. FDA Accepts sNDA for Arcutis’ Roflumilast Cream 0.05% (Zoryve) for Children Aged 2 to 5 with Mild-to-moderate AD; PDUFA Date Set
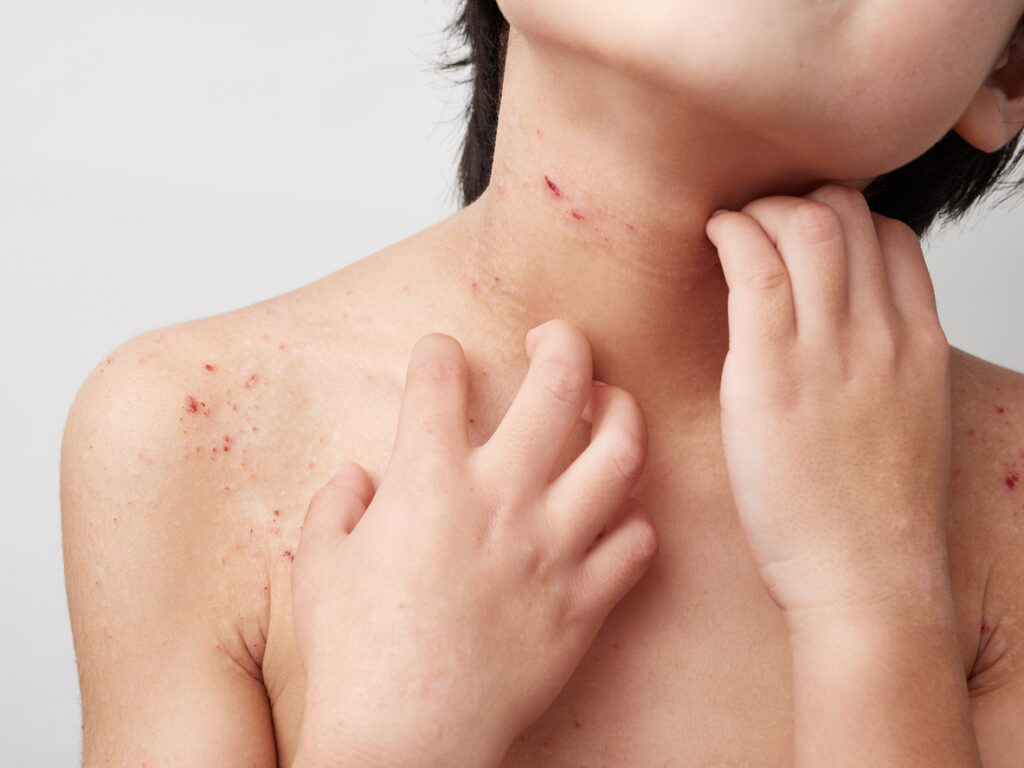
The U.S. Food and Drug Administration (FDA) accepted Arcutis’ Supplemental New Drug Application (sNDA) for roflumilast cream 0.05% (Zoryve) for the treatment of children aged 2 to 5 with mild-to-moderate atopic dermatitis (AD). The Prescription Drug User Fee Act (PDUFA) target action date is set for October 13, 2025. “In clinical trials, investigational ZORYVE cream […]
